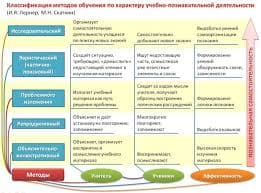
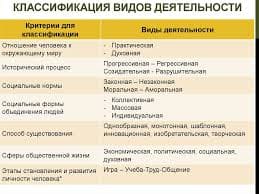
1. What is organic chemistry?
2. Why are there more organic compounds than inorganic ones?
3. What is the most important source of organic compounds at the present time?
4. What sugars does the author mean?
5. Why are carbon compounds so important?
6. What was the source of organic chemicals in the past?
7. What chemical did Wohler prepare in the laboratory?
8. What else can Wohler be credited with?
9. What do you know about petroleum?
10. It's written in the text: "Nowadays, however, we use the term "organic compounds" to mean carbon compounds, there being some exceptions to the rule." What are the exceptions?
11. Why does carbon have so many more compounds than other elements?
12. Why is number of possible hydrocarbons enormous?
4. Choose a word in the columns (a) or (b) that has nearly the same meaning as the italicized word:
| A | B | |
| 1. to learn | to know | to find out |
| 2. essential | significant | fundamental |
| 3. man-made | important | artificial |
| 4. to consider | to think | to weigh |
| 5. to convert | to study | to transform |
| 6. to manufacture | to do | to produce |
| 7. to join | to combine | to associate |
| 8. separated | divisible | separated |
| 9. directly | spontaneously | immediately |
| 10. nowadays | at present | at some time |
| 11. exception | elimination | deviation |
| 12. reason | cause | intuition |
| 13. rule | law | instruction |
| 14. marine | naval | oceanic |
5. Translate the following text into English:
Ще на початку ХІХ століття хіміки не могли не дивуватися тому, що органічні речовини при нагріванні легко перетворюються в неорганічні. Учені того часу, які мали справу зі звичайними сполуками та користувалися звичайними методами, не могли синтезувати органічні сполуки.
|
|
|
1828 року німецький хімік, учень Берцеліуса, Фрідріх Веллер, шляхом нагрівання ціанату амонію (ammonium cyanate) отримав сечовину. Повторивши дослід декілька разів, Веллер зрозумів, що може перетворювати неорганічні сполуки в органічні. Він повідомив Берцеліуса про своє відкриття, і його вчитель не міг не погодитися з тим, що Веллер має рацію.
TEXT 13
CARBON
Learn the new vocabulary:
| to constitute | складати |
| relatively small amount | відносно мала кількість |
| Earth’s crust | земна кора / поверхня |
| approximately | приблизно, майже |
| aid | допомога |
| to differ | відрізнятися |
| strikingly | разюче, дуже |
| conductor | провідник |
| on the contrary | навпаки |
| transparent | прозорий |
| X-rays | рентгенівські промені |
| to enable | давати можливість |
| to share electrons | ділитися електронами / віддавати електрони |
| to be arranged | розміщуватися |
| hence | звідси, відповідно |
| giant | гігантський |
| to account (for) | пояснювати |
| to furnish bonds | забезпечувати зв’язки |
1. Read and translate the text:
Carbon is to be ranked along with hydrogen and oxygen as one of the most important of all the elements to man. Carbon occurs in nature as a free element and in many compounds. It constitutes only about 0.03 percent of the Earth's crust, but this relatively small amount of the element is of great importance. Its importance is indicated by the 300,000 or more compounds of the element which exist naturally or which have been prepared. It is proved that this number is approximately ten times the number of compounds of all the other elements put together.
For a long time it was believed that these compounds might have never been produced except with the aid of organic life, in other words, by living plants and animals. For this reason they were called organic compounds.
It is known that carbon occurs in two crystalline forms which differ strikingly by their properties. Graphite is black, soft, a good conductor of electricity. Diamond, on the contrary, is colorless and transparent, the hardest of known substances, a non-conductor of electricity. It is the crystal structure, as determined by X-rays, which gives an explanation of this contrast of properties. The four valence electrons of each carbon atom enable it, by sharing electrons with four of its neighbors, to be linked with them in a covalent union.
|
|
|
It may be shown by X-rays examination that in the diamond the four nearest neighbors of each carbon atom are symmetrically arranged about it in space. All atoms in a diamond are thus firmly linked together hence the whole crystal acts as a giant molecule. Thus we account for the extreme hardness of the diamond, its high melting point, and its failure to dissolve in any solvent.
On the other hand, it is found that graphite possesses parallel planes of atoms, and each is at a considerable distance from its neighbors. Each carbon atom in graphite has three nearest neighbors and they all are present in its own plane. Only three of the four valence electrons of each atom are needed for furnishing bonds with these nearest neighbors and the fourth is available for producing a bond with a neighboring plane. A certain portion of the electrons in graphite are relatively free to move as it is true of metals. Hence, graphite is a conductor of electricity.
2. Read the text again, divide it into logical parts and entitle them.
Write out of the text all the sentences expressing the main idea(s) of each logical part.
 2018-02-13
2018-02-13 545
545