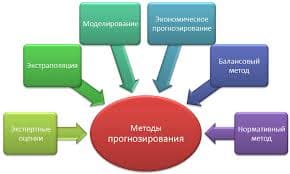
| Электрохимическая система | 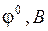
|
MnO2 + 4H+ + 2  = Mn2+ + 2H2O = Mn2+ + 2H2O
| 1,23 |
MnO  + 8H+ + 5 + 8H+ + 5  = Mn2+ + 4H2O = Mn2+ + 4H2O 
| 1,52 |
NO 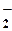 + 2H+ + + 2H+ +  = NO (г) + H2O = NO (г) + H2O
| 1,0 |
I2 + 2  = 2I = 2I 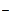
| 0,5355 |
MnO2 + 4H+ + 2  = Mn2+ + 2H2O = Mn2+ + 2H2O
| 1,23 |
S2- - 2  = S0 = S0
| -0,48 |
Cr2O 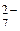 + 14H+ + 6 + 14H+ + 6  = 2Cr3+ + 7H2O = 2Cr3+ + 7H2O
| 1,33 |
Fe2+ -  = Fe3+ = Fe3+
| 0,771 |
ClO   + 6H+ + 6 + 6H+ + 6  = Cl = Cl  + 3H2O + 3H2O
| 1,45 |
SO 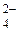 + 8H+ + 8 + 8H+ + 8  = S2- + 4H2O = S2- + 4H2O
| 0,149 |
SO2 + 2H2O - 2  = SO = SO  + 4H+ + 4H+
| 0,138 |
NO  + 2H+ + + 2H+ +  = NO2 (г) + H2O = NO2 (г) + H2O
| 0,80 |
NO  + 4H+ + 3 + 4H+ + 3  = NO (г) + 2H2O = NO (г) + 2H2O
| 0,957 |
2Br 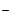 - 2 - 2  = Br2 = Br2
| 1,07 |
MnO  + 1 + 1  = MnO = MnO 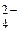
| 0,564 |
2Cl 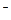 - 2 - 2  = Cl2 = Cl2
| 1,359 |
NO  + 3H+ + 2 + 3H+ + 2  = HNO2 + H2O = HNO2 + H2O
| 0,960 |
PbO2 + 4H+ + 2  = Pb2+ + 2H2O = Pb2+ + 2H2O
| 1,682 |
2IO  + 12H+ + 10 + 12H+ + 10  = I2 + 6H2O = I2 + 6H2O
| 1,195 |
Ti4+ +  = Ti3+ = Ti3+
| -0,04 |
Pb2+ + 2  = Pb0 = Pb0
| -0,126 |
Cr3+ + 3  = Cr0 = Cr0
| -0,744 |
Mn2+ + 2  = Mn0 = Mn0
| -1,180 |
Co2+ + 2  = Co0 = Co0
| -0,277 |
Fe2+ + 2  = Fe0 = Fe0
| -0,44 |
Cd2+ + 2  = Cd0 = Cd0
| -0,4029 |
Cu2+ + 2  = Cu0 = Cu0
| 0,337 |
Sn2+ + 2  = Sn0 (белый) = Sn0 (белый)
| -0,136 |
Au3+ + 3  = Au0 = Au0
| 1,498 |
| Окончание прилож. 3 | |
Mg2+ + 2  = Mg0 = Mg0
| -2,363 |
Zn2+ + 2  = Zn0 = Zn0
| -0,763 |
Al3+ + 3  = Al0 = Al0
| -1,662 |
Ag+ +  = Ag0 = Ag0
| 0,799 |
Sb3+ + 3  = Sb0 = Sb0
| 0,24 |
Pd2+ + 2  = Pd0 = Pd0
| 0,987 |
Sn4+ + 2  = Sn2+ = Sn2+
| 0,009 |
Cr2+ + 2  = Cr0 = Cr0
| -0,913 |
Ni2+ + 2  = Ni0 = Ni0
| -0,25 |
In3+ + 3  = In0 = In0
| -0,343 |
Fe3+ + 3  = Fe0 = Fe0
| -0,036 |
2H+ + 2  = H2 = H2
| |
Li+ +  = Li0 = Li0
| -3,045 |
Примерная тематика бакалаврских выпускных квалификационных работ по направлению подготовки 030300 психология,
 2014-10-30
2014-10-30 222
222