The albedos of planets, satellites and asteroids can be used to infer much about their properties. The study of albedos, their dependence on wavelength, lighting angle ("phase angle"), and variation in time comprises a major part of the astronomical field of photometry. For small and far objects that cannot be resolved by telescopes, much of what we know comes from the study of their albedos. For example, the absolute albedo can indicate the surface ice content of outer Solar System objects, the variation of albedo with phase angle gives information about regolith properties, whereas unusually high radar albedo is indicative of high metal content in asteroids.
Enceladus, a moon of Saturn, has one of the highest known albedos of any body in the Solar System, with 99% of EM radiation reflected. Another notable high-albedo body is Eris, with an albedo of 0.96. Many small objects in the outer Solar System and asteroid belt have low albedos down to about 0.05. A typical comet nucleus has an albedo of 0.04. Such a dark surface is thought to be indicative of a primitive and heavily space weathered surface containing some organic compounds.
The overall albedo of the Moon is around 0.12, but it is strongly directional and non-Lambertian, displaying also a strong opposition effect. Although such reflectance properties are different from those of any terrestrial terrains, they are typical of the regolith surfaces of airless Solar System bodies.
In detailed studies, the directional reflectance properties of astronomical bodies are often expressed in terms of the five Hapke parameters which semi-empirically describe the variation of albedo with phase angle, including a characterization of the opposition effect of regolith surfaces.
- Explain the following formula:
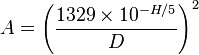
- Write definitions of the following terms: planet, asteroid, comet nucleus, organic compounds, opposition effect.
Физика және математика кафедрасының отырысында қаралып, бекітілген.
Хаттама № «7» __18_____ ____03______ 2015 ж.
Кафедра меңгерушісі:____________ Кульжумиева А.А.
Факультеттің ОӘК отырысында қаралып, бекітілген.
Хаттама № «7» __19____ ____03_____ 2015 ж.
Факультеттің ОӘК төрағасы: _________ Иксебаева Ж.С.
М. Өтемісов атындағы Батыс Қазақстан мемлекеттік университеті
Факультет: физика-математика
Кафедра: физика және Физика
Шифр, мамандық: 5В011000 – Физика
Пән: Кәсіби бағытталған шетел тілі
Билет №7
- Read and retell the text:
Atom
An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is made up of neutral or ionized atoms. Atoms are very small; typical sizes are around 100 pm (a ten-billionth of a meter, in the short scale). However, atoms do not have well defined boundaries, and there are different ways to define their size which give different but close values.
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and typically a similar number of neutrons (none in hydrogen-1). Protons and neutrons are called nucleons. Over 99.94% of the atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, that atom is electrically neutral. If an atom has more or less electrons than protons, then it has an overall negative or positive charge, respectively, and it is called an ion.
Electrons of an atom are attracted to the protons in an atomic nucleus by this electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by a different force, the nuclear force, which is usually stronger than the electromagnetic force repelling the positively charged protons from one another. Under certain circumstances the repelling electromagnetic force becomes stronger than the nuclear force, and nucleons can be ejected from the nucleus, leaving behind a different element: nuclear decay resulting in nuclear transmutation.
- Explain the following scheme:

- Write definitions of the following terms: chemical element, ion, nuclear force, electrons, electric charge.
Физика және математика кафедрасының отырысында қаралып, бекітілген.
Хаттама № «7» __18_____ ____03______ 2015 ж.
Кафедра меңгерушісі:____________ Кульжумиева А.А.
Факультеттің ОӘК отырысында қаралып, бекітілген.
Хаттама № «7» __19____ ____03_____ 2015 ж.
Факультеттің ОӘК төрағасы: _________ Иксебаева Ж.С.
М. Өтемісов атындағы Батыс Қазақстан мемлекеттік университеті
Факультет: физика-математика
Кафедра: физика және Физика
Шифр, мамандық: 5В011000 – Физика
Пән: Кәсіби бағытталған шетел тілі
Билет №8
- Read and retell the text:
 2015-06-05
2015-06-05 456
456