Fluorescence is particularly favored in molecules with rigid structures. The table below compares the quantum efficiencies of fluorine and biphenyl which are both similar in structure that there is a bond between the two benzene group. The difference is that fluorene is more rigid from the addition methylene bridging group. By looking at the table below, rigid fluorene has a higher quantum efficiency than unrigid biphenyl which indicates that fluorescence is favored in rigid molecules.
| Compound | Structure | Quantum Efficiency |
| Fluorene | 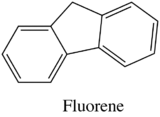
| 1.0 |
| Biphenyl | 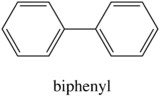
| 0.2 |
Table 7. Quantum Efficiencies in Rigid vs. Nonrigid structures
This concept of rigidity was used to explain the increase in fluorescence of organic chelating agent when the compound is complexed with a metal ion. The fluorescence intensity of 8-hydroxyquinoline is much less than its zinc complex.
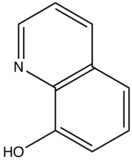 vs
vs 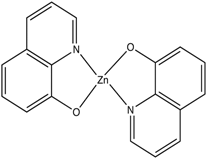
8-hydroxyquinoline 8-hydroxyquinoline with Zinc complexed
The explanation for lower quantum efficiency or lack of rigidity in caused by the enhanced internal conversion rate (kic) which increases the probability that there will be radiationless deactivation. Nonrigid molecules can also undergo low-frequency vibration which accounts for small energy loss.
 2015-08-21
2015-08-21 350
350