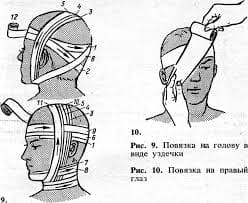
A periodic table is a tabular display of the different elements. The elements are structured as per their properties and atomic structure. This table helps in easy understanding of the elements and also helps in knowing the properties of the elements and how different are they from each other.
Do you know how was Periodic Table discovered?
In 1789, Antoine Lavoisier published a list of 33 chemical elements. He grouped the elements as gas, non-metals, metals and earths. For years together, the chemists spent time in researching for a better and more precise classification of the elements.
In 1829, Johann Wolfgang Dobereiner observed that many of the elements could be grouped together based on their chemical properties. He also found that when such elements were grouped together as per their atomic weight, the second member of each group was roughly the average of the first and the third element in that group.
In 1858, August Kekule discovered that some elements had the capacity to bond with others and this type of grouping was called as valency. The periodic table was then revised on the basis of valency wherein elements that could bond with each other were grouped together.
In 1869, Russian chemistry professor Dmitri Ivanovich Mendeleev revised the periodic table again. He left gaps while grouping similar chemical elements. He did this deliberately to accommodate the future elements with similar properties that can fit the group in the table. He prepared the periodic table on the basis of increasing atomic numbers.
Post this creation of periodic table, the chemists were able to fill the table with elements that were discovered in later stages in the gaps left by Mendeleev. Mendeleev's structure of periodic table was considered as the correct and a better way and was followed by the chemists from all over the world. In this way our concise and precise periodic table evolved over the years.
Know the benefits of a periodic table
The periodic table is based on the atomic numbers of the chemical elements. The table shows why the elements in the same group have similar properties. The periodic table is orderly and follows the basic electronic structure to categorise the elements.
The periodic table is simple, systematic and helps in easy understanding and remembering the properties of the various chemical elements.
The Periodic Table
 2015-09-06
2015-09-06 671
671