In chemistry and physics, the Avogadro constant (symbols: L, N A) is the number of constituent particles, usually atoms or molecules, that are contained in the amount of substance given by one mole. Thus it is the proportionality factor that relates the molar mass of a material to its mass. It has the dimension of reciprocal amount of substance. Avogadro's constant has the value 7023602214129000000♠6.02214129(27)×1023 mol−1 in the International System of Units (SI).
Previous definitions of chemical quantity involved Avogadro's number, a historical term closely related to the Avogadro constant but defined differently: Avogadro's number was initially defined by Jean Baptiste Perrin as the number of atoms in one gram-molecule of atomic hydrogen, meaning (in modern terminology) one gram of (atomic) hydrogen. It was later redefined as the number of atoms in 12 grams of the isotope carbon-12 (12C) and still later generalized to relate amounts of a substance to their molecular weight. For instance, to a first approximation, 1 gram of hydrogen element (H), which has a mass number of 1 (atomic number 1), has 7023602190000000000♠6.022×1023 hydrogen atoms. Similarly, 12 grams of 12C, with the mass number of 12 (atomic number 6), has the same number of carbon atoms, 7023602190000000000♠6.022×1023. Avogadro's number is a dimensionless quantity and has the numerical value of the Avogadro constant given in base units.
The Avogadro constant is fundamental to understanding both the makeup of molecules and their interactions and combinations. For instance, since one atom of oxygen will combine with two atoms of hydrogen to create one molecule of water (H2O), one can similarly see that one mole of oxygen (7023602190000000000♠6.022×1023 of O atoms) will combine with two moles of hydrogen (2 × 7023602190000000000♠6.022×1023 of H atoms) to make one mole of H2O.
- Explain the following formula:
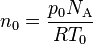
- Write definitions of the following terms: gas, ideal gas, molar mass,Mole, SI units.
Физика және математика кафедрасының отырысында қаралып, бекітілген.
Хаттама № «7» __18_____ ____03______ 2015 ж.
Кафедра меңгерушісі:____________ Кульжумиева А.А.
Факультеттің ОӘК отырысында қаралып, бекітілген.
Хаттама № «7» __19____ ____03_____ 2015 ж.
Факультеттің ОӘК төрағасы: _________ Иксебаева Ж.С.
М. Өтемісов атындағы Батыс Қазақстан мемлекеттік университеті
Факультет: физика-математика
Кафедра: физика және Физика
Шифр, мамандық: 5В011000 – Физика
Пән: Кәсіби бағытталған шетел тілі
Билет №12
- Read and retell the text:
 2015-06-05
2015-06-05 412
412