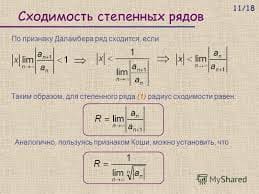
Electrolysis is a process by which a chemical reaction is carried out by means of the passage of an electric current. The electrical energy enters and leaves the electrolytic medium through electrodes, which ordinarily are pieces1 of metal. The electrode where electrons enter the solution is the cathode; the electrode where the electrons leave is the anode. Negatively charged ions (anions) are attracted to the anode; positively charged ions (cations) are attracted to the cathode.
The process is generally used as a method of depositing2 metals from solution.
The relation between the quantity of material undergoing reaction and the quantity of electricity usedin this reaction was discovered by Faraday.
Пояснения к тексту
1. pieces— кусочки
2. depositing—осаждения, выделения
X. Переведите, пользуясь словарем:
 2015-09-07
2015-09-07 616
616