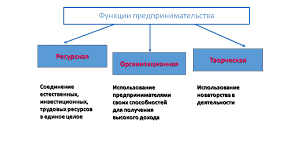
Vacuum distillation. The principles of vacuum distillation resemble those of fractional distillation, and, except that larger diameter columns are used to maintain comparable vapour velocities at the reduced pressures, the equipment is also similar. The vacuum is produced by steam ejectors in vacuum distillation. Components that are less volatile can be distilled without raising the temperature to the range at which cracking occurs, as it would at atmospheric pressure. Firing conditions in the furnace are adjusted so that the oil temperature does not exceed about 400 °C (750 °F). The residue after vacuum distillation is asphalt, or bitumen.
Superfractionation. Superfractionation is an extension of fractional distillation employing columns with a much larger number of trays (e.g.,100) and reflux ratios exceeding 5:1. With such equipment it is possible to obtain fractions containing only a few hydrocarbons or even to separate pure compounds. By this method, isopentane of over 90 percent purity is produced for aviation gasoline; isohexane and isoheptane concentrates are also prepared for the same purpose. These isoparaffins have much higher octane numbers than the corresponding normal paraffins.
Other methods. Absorption and stripping. Absorption and stripping are processes used to obtain valuable light products such as propane/propylene and butane/butylenes from the gasoline vapours that pass out of the top of the fractionating tower. In the absorption process, gasoline vapours are bubbled through an absorption oil such as kerosene or heavy naphtha in equipment resembling a fractionating column. The light products dissolve in the oil while dry gases such as hydrogen, methane, ethane, and ethylene pass through undissolved. Absorption is more effective under pressures of about 7 to 11 kilograms per square centimetre (100 to 150 pounds per square inch) than it is at atmospheric pressure.
The light products are separated from the absorption oil in the stripping process. The solution of absorption oil and light products is boiled by steam and passes to a stripping column, where the light product vapours pass upward and are recovered by condensation by water-cooling under pressure. The unvaporized oil passes from the base of the column for reuse.
Solvent extraction. Solvent-extraction processes are used primarily for the removal of constituents that would have an adverse effect on the performance of the product in use. The quality of kerosene is improved by the extraction of aromatic compounds that burn with a smoky flame (these compounds can be used to advantage in tractor fuels).
Another important operation is the removal of heavy aromatic compounds from lubricating oils. Removal improves the viscosity-temperature relationship of the oil, extending the temperature range over which satisfactory lubrication is obtained. The usual solvents for extraction of lubricating oil are furfural and phenol. Other solvents are dichloroethyl ether, nitrobenzene, and a mixture of liquid propane and cresylic acid.
Adsorption. Certain highly porous, solid materials have the ability to select and adsorb specific types of molecules, thus separating them from other types. Silica gel is used in this way to separate aromatics from other hydrocarbons, and activated charcoal is used to remove liquid components from gases.
Adsortion is thus somewhat analogous to the process of absorbtion with an oil, although the principles are different. Layers of the adsorbed material only a few molecules thick are formed on the extensive interior surface of the adsorbent; this interior surface may amount to several acres per pound of material.
Recent years have brought new developments in the use of adsorbents of a very selective nature called molecular sieves. Molecular sieves are produced by the dehydration of naturally occurring or synthetic zeolites (crystalline alkali-metal aluminosilicates). The dehydration leaves intercrystalline cavities that have pore openings of definite size, depending upon the alkali metal of the zeolite. Under adsorptive conditions normal paraffin molecules can enter the crystalline lattice and be selectively retained, whereas the other molecules are excluded. This principle is used commercially for the removal of normal paraffins from gasoline fuels, thus increasing their octane number. The use of molecular sieves has also been extended to the separation of hydrocarbons of higher molecular weight.
Thermal diffusion. When a mixture of hydrocarbons is passed through a narrow gap, of the order of 0.25 millimetre (0.01 inch), between hot and cold surfaces, some of the constituents concentrate near the hot surface and others near the cold. The phenomenon, known as thermal diffusion, is not clearly understood, but it is believed that separation occurs as a result of differences in the shapes of the molecules. Though this process has been applied in the laboratory as an analytical tool, it is unlikely to find much use in industry as the thermodynamic efficiency is low.
Crystallization. The crystallization of wax from lubricating oil fractions is essential to make the oils suitable for use. A solvent (e.g., a mixture of benzene and methyl ethyl ketone) is first added to the oil and the solution chilled to about -20 °C (-5 °F). The function of the benzene is to keep the oil in solution and maintain its fluidity at low temperatures, whereas the methyl ethyl ketone acts as a wax precipitant. Rotary filters are used to filter off the wax crystals on a specially woven canvas cloth stretched over a perforated cylindrical drum. A vacuum, maintained within the drum, sucks the oil into it. The wax crystals are removed from the cloth by metal scrapers, after washing with solvent to remove traces of oil. The solvents are later distilled from the oil and reused.
 2015-10-13
2015-10-13 467
467