Matter is the stuff of life — literally. Every living thing is made of matter. In order to grow, living things must get more matter to build new structures. When living things die, be they plants or animals, microbes such as bacteria and fungi digest the dead matter and recycle it so that other living things can use it again. In fact, pretty much all the matter on Earth has been here since the planet formed 4.5 billion years ago; it has just been recycled since then. So, the stuff that makes up your body may once have been part of Tyrannosaurus rex, a butterfly, or even a bacterium.
Matter could be invisible: what looks like nothing but is really something? Air! Earth’s atmosphere may seem like nothing, but it’s made of gases such as nitrogen, carbon dioxide, and oxygen. These gases interact with living things in many ways. Plants, for example, take in carbon dioxide to make food and then use that food to build their structures. It’s hard to believe, but the tallest tree in the redwood forest grows and grows from the result of invisible carbon dioxide gas being taken in and incorporated into the body of the tree. Obviously the redwood tree takes up space and has mass, but those invisible carbon dioxide molecules are matter too.
Ex. 2. Read the following Latin names of animals in English.
Alces alces
Antilocapra americana
Arvicola richardsoni
Canis latrans
Canis lupus
Castor canadensis
Cervus elaphus
Clethrionomys gapperi
Eptesicus fuscus
Erethizon dorsatum
Eutamias amoenus
Eutamias minimus
Felis concolor
Felis lynx
Felis rufus
Glaucomys sabrinus
Lepus americanus
Lontra canadensis
Marmota caligata
Martes americana
Martes pennanti
Mephitis mephitis
Microsorex hoyi
Microtus pennsylvanicus
Ex. 3. Fill the gaps with the words from the list below. Be ready to interpret the text.
Space, gases, liters, solids, grams, mass, weigh, gravity, volume, liquids.
Following are a few facts you should know about matter:
✓ Matter takes up …. Space is measured in volume, and volume is measured in liters (L).
✓ Matter has mass. … is the term for describing the amount of matter that a substance has. It’s measured in … (g). Earth’s … pulls on your mass, so the more mass you have, the more you ….
✓ Matter can take several forms. The most familiar forms of matter are solids, liquids, and gases. … have a definite shape and size, such as a person or a brick. … have a definite volume. They can fill a container, but they take the shape of the container that they fill. … are easy to compress and expand to fill a container.
Ex. 4. Find the definitions for the words from the list below. Be ready to interpret the definitions.
Atom, negative ions, quarks, subatomic particles, leptons, protons, mesons, electrons, positive ions, neutrons
1. The smallest particle of a chemical element that can exist.
2. A particle smaller than an atom (e.g., a neutron) or a cluster of such particles (e.g., an alpha particle).
3. A stable subatomic particle occurring in all atomic nuclei, with a positive electric charge equal in magnitude to that of an electron.
4. A subatomic particle of about the same mass as a proton but without an electric charge, present in all atomic nuclei except those of ordinary hydrogen.
5. A stable subatomic particle with a charge of negative electricity, found in all atoms and acting as the primary carrier of electricity in solids.
6. Any of a number of subatomic particles carrying a fractional electric charge, postulated as building blocks of the hadrons.
7. A subatomic particle which is intermediate in mass between an electron and a proton and transmits the strong interaction that binds nucleons together in the atomic nucleus.
8. A subatomic particle, such as an electron, meson, or neutrino, which does not take part in the strong interaction.
9. An electrically charged atom or group of atoms formed by the loss of gain of one or more electrons as a cation which is created by electron loss.
10. An electrically charged atom or group of atoms formed by the loss of gain of one or more electrons as an anion which is created by electron gain.
Ex. 5. Translate the following text into English.
Усі хімічні речовини складаються з частинок, класифікація яких в хімії є досить складною. Хімічні перетворення пов’язують насамперед із такими частинками як атом, молекула, ядро, електрон, протон, нейтрон, йон.
Вважають, що атом є найменшою хімічною частинкою речовини. Атом – це електронейтральна система взаємодіючих елементарних частинок, що складається з позитивно зарядженого ядра та негативно заряджених електронів. Ядро атома утворене протонами, які мають позитивний заряд, та нейтронами, які не мають заряду. Експериментально встановлено, що атом є електронейтральним, оскільки позитивний заряд усіх протонів компенсує негативний заряд усіх електронів в атомі. Отже, кількість електронів в атомі дорівнює кількості протонів у його ядрі. Величина заряду ядра атома дорівнює кількості протонів в атомі.
Наприклад, в атомі оксигену вісім протонів. Отже, величина заряду ядра його атома плюс вісім. А навколо ядра розташовано вісім електронів.
Ex. 6. Translate the following text into your native language.



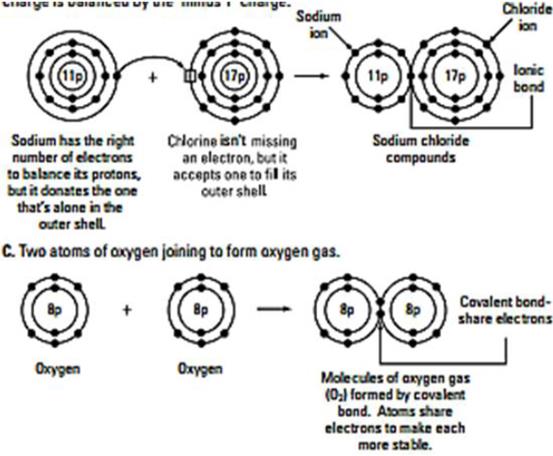
Ex. 7. Read the following chemical reactions in English.
H2+O2=H2O
Al+S=Al2S3
AgCl+Na2S=Ag2S+NaCl
ZrCl4=ZrCl3+ZrCl2+ZrCl+Cl2
NaOH+Cl2+Br2=NaBrO3+NaCl+H2O
2As + 3Cu = Cu3As2
2NO + 4Cu = N2 + 2Cu2O
2Cu + 2H2SO4+ O2= 2CuSO4 + 2H2O
Ex. 8. Translate the following text into your native language.
 2015-10-13
2015-10-13 377
377