Lecture No. 3
Carbon nanotubes.Properties and applications.
Graphene.Properties and chemistry.
Carbon nanoporous materials (CNPM). Method for producing CNPM.
Objectives:
Ø To describe carbon nanotubes, graphene and nanoporous materials.
Ø To explain properties of carbon based materials (CBM) and the most important applications.
Ø To elucidatesome methods for producing CBM.
Carbon is known for its large variety of allotropic forms, structural and topological characteristics which are the fundamental cause of several unique physical properties and phenomena associated with carbon materials. We were studying fullerenes and they are the third most stable form of carbon, after diamond and graphite. Also, we studied different types of fullerenes. Among them are carbon nanotubes and today we are going to study this type of fullerenes.
At the end of the activity students will be able to fulfil the suggested objective.
Carbon nanotubes
In chemistry, nanotubes are called tubular structures whose diameter is of the order of nanometer (nm)and a length of several tens of microns. They have more than a million atoms of carbon. In the walls of the tube the carbon atoms are located at the vertices of regular hexagons.
There are nanotubes of many materials, such as silicon or boron nitride, but generally the term is applied to carbon nanotubesor CNTs (Carbon Nanotubes) (Fig.1)


Fig. 1. Carbon nanotubes
Carbon nanotubes are an allotropic form of carbon, such as diamond, graphite or fullerenes. Its structure can be considered as coming from a sheet of graphite wrapped around itself. Depending on the degree of winding and the way the original sheet is formed, the result can lead to nanotubes of different diameter and internal geometry.
The nanotubes formed as if the corners of a folio were united by their ends forming a tube, are called monotube nanotubes, or SWNTs (Single-Walled Nanotubes) (Fig. 2)


Most single wall nanotubes have a diameter of about 1 nanometer, with a tube length that can be many thousands of times more.
There are also nanotubes whose structure resembles that of a series of concentric tubes, including inside each other as "matriuska dolls" and logically with increasing thicknesses from the center to the periphery. The latter are multilayered nanotubes or MWNTs (Multi-walledNanotubes)and can be tens of nanometers in diameter, made from numerous tubes. Both the single-wall and multiwall types occur commonly.
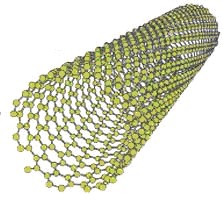

Fig. 3. Single-Walled Nanotubes and Multi-WalledNanotubes
They are being actively studied, such as fullerenes, because of their fundamental interest in chemistry and their technological applications.
Characteristics of nanotubes
Nanotubes are 100 thousand times thinner than a human hair, but it is a very durable material. Nanotubes is 50 to 100 times stronger than steel and has six times lower density. Young's modulus – the resistance of material to deformation of nanotubes is twice that of conventional carbon fibers. This suggests that nanotubes are not only durable, but also flexible, and resemble in their behavior is not brittle straw, and the hard rubber tube. Under the action of mechanical stresses exceeding a critical, nanotubes do not "break", not "break" and rebuild.
Currently the maximum length of the synthesized nanotubes is tens and hundreds of microns–which, of course, is very large on the atomic scale, but too small for everyday use. However, the length of the resulting nanotubes is gradually increasing-scientists are now already close to a centimeter milestone. The obtained multilayer nanotubes with a length of 4 mm.
They have unique electrical, magnetic, optical properties. For example, depending on the specific schema of folding the graphite plane (chirality), the nanotubes can be conductors and semiconductors of electricity. Electronic properties of nanotubes can be purposefully changed by introducing inside the tubes atoms of other substances.
The emptiness inside of fullerenes and nanotubes has long attracted the attention scientists. The experiments showed that if the inside of the fullerene to embed an atom some substances (this process is called "intercalation", i.e. "introduction"), it can change its electrical properties, and even turn an insulator into a superconductor. Is it possible in the same way, to modify the properties of nanotubes? It turns out, Yes. Scientists could put nanotubes inside a whole chain of fullerenes with already embedded in them by the atoms of gadolinium. The electrical properties of this unusual structure are very different from the properties of simple, hollow nanotubes, and nanotubes with properties of empty fullerenes inside. It is interesting to note that for such compounds developed specific chemical designation. The above-described structure is written as Gd@C60@SWNT, which means "Gd inside C60 inside single-layer carbon nanotubes (Single Wall Nanotube)".
They have been found to have higher mechanical strength and greater flexibility than carbon fibers.
The nanotubes are now the elements with greater physical resistance found on the earth, in terms of elasticity, consequence of the type of covalent bonds between the carbons of the nanotube.
Carbon nanotubes are the strongest fibers known. A single perfect nanotube is 10 to 100 times stronger than steel.
Multilayer nanotubes, if nested accurately, can be moved within each other with little friction. This property has great applications in molecular nanotechnology (to place each atom justin its place)

Fig. 4. Multilayer nanotubes.
All nanotubes are good thermal conductors, possessing a special property called "ballistic conduction". It is predicted that nanotubes will be able to transmit about 20 times more heat than metals like copper.
Because of its nanodimensions, the transport of electrons in a carbon nanotube will occur like quantum form and the electrons can only be propagated through the axis of the nanotube. Due to this special transport property carbon nanotubes are usually referred to as one-dimensional.
Table 1. A summary of the properties of nanotubes
| Property | Single wall nanotubes | By comparison with other substances or elements |
| Size | 0.6 to 1.8 nanometers in diameter | Electronic beam lithography can create 50 nm wide lines. |
| Density | 1.33 to 1.40 g/cm3 | Aluminum has a density of 2.7 g/cm3 |
| Tensile strength | 45000 MPa | High strength steel alloys break down to about 2 000 MPa |
| Elasticity | They can bend at large angles and return to their original state without damage. | The metals and carbon fibers fracture at similar stresses. |
| Current carrying capacity | Estimated in 1 billion amperes per square centimeter | Copper wires are melted at about a million amperes per square centimeter approximately. |
| Field emission | Matches can be activated with 1 to 3 volts if the electrodes are spaced one micron. | Molybdenum tips require fields of 50 to 100 V/m and have very limited lifetimes. |
| Heat Transmission | It is predicted to be as high as 6,000 W per meter per kelvin at room temperature. | The almost pure diamond transmits 3.320 W/mK |
| Thermal stability | Stable still at 2,800 °C in vacuum, and 750 °C in air. | The metal wires in microchips melt between 600 and 1000 °C. |
 2017-11-30
2017-11-30 667
667