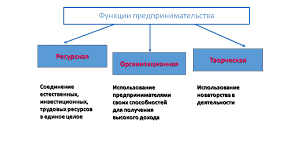
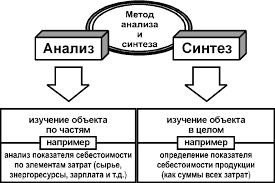
| Название и символ элемента | Электродный процесс | Стандартный электродный потенциал j°, В |
|
Азот N | 3N2 + 2H+ + 2e – = 2NH3 | -3,1 |
| N2 + 4H2O + 2e– = 2NH2ON + 2ON – | -3,04 | |
| N2 + 2H2O + 4H + 2e– = (2NH2OH) H+ | -1,87 | |
| N2 + 4H2O + 4e– = N2H4 + 4OH – | -1,16 | |
| N2 + 5H+ + 4e– = (N2H4) H+ | -0,23 | |
| N2 + 6H+ + 6e– = 2NH3 | +0,057 | |
| N2 + 8H+ + 6e– = NH4+ | +0,275 | |
| N2O + 10H+ + 8e– = 2NH4+ + H2O | +0,647 | |
| 2NO + 2H+ + 2e– = H2N2O2 | +0,71 | |
| HNO2 + 6H+ + 6e– = NH3 + 2H2O | +0,755 | |
| HNO3 + H+ + e– = NO2 + H2O | +0,755 | |
| NO3 – + 2H+ + e– = NO2 + H2O | +0,755 | |
| 2HNO3 + 2H+ + 2e– = N2O4 + 2H2O | +0,803 | |
| 2NO3– + 4H+ + 2e– = N2O4 + 2H2O | +0,803 | |
| NO3– + 2H+ + 2e– = NO2– + H2O | +0,835 | |
| NO + 6H+ + 5e– = NH4+ + H2O | +0,386 | |
| HNO2 + 7H+ + 6e– = NH4+ + 2H2O | +0,864 | |
| NO3– + 10H+ + 8e– = NH4+ + 3H2O | +0,87 | |
| HNO3– + 2H+ + 2e– = HNO2 + H2O | +0,934 | |
| HNO3 + 3H+ + 3e– = NO + 2H2O | +0,957 | |
| NO3– + 4H+ + 3e– = NO + 2H2O | +0,957 | |
| HNO2 + H+ + e– = NO + H2O | +1,004 | |
| NO2 + 2H+ + 2e– = NO + H2O | +1,049 | |
| NO2 + 2H+ + e– = HNO2 | +1,093 | |
| HNO3 + 8H+ + 8e– = N2O + 5H2O | +1,116 | |
| 2NO3 – + 10H+ + 8e– = N2O + 5H2O | +1,116 | |
| 2HNO3 + 10H+ + 10e– = N2 + 6H2O | +1,246 | |
| 2HNO2 + 4H+ + 4e– = N2O + 3H2O | +1,297 | |
| 2NO2 + 8H+ + 8e– = N2 + 4H2O | +1,363 | |
| 2HNO2 + 6H+ + 6e– = N2 + 4H2O | +1,454 | |
| 2NO + 4H+ + 4e– = N2 + 2H2O | +1,678 | |
| N2O + 2H+ + 2e– = N2 + H2O | +1,766 | |
| H2N2O2 + 2H+ + 2e– = N2 + 2H2O | +2,65 | |
|
Алюминий Al | [AlF6] 3– + 3e– = Al + 6F – | - 2,07 |
| Al 3+ + 3e– = Al | -1,663 | |
| Al3+ + 3H+ + 3e– = Al + 3H2O | -1,471 | |
| AlO2 – + 4H – + 3e– = Al + 2H2O | -1,262 | |
| Барий Ba | Ba2+ + 2e – = Ba | -2,905 |
|
Бериллий Ве | Be 2+ + 2e– = Be | -1,847 |
| Be(OH)2 + 2H+ + 2e– = Be + 2H2O | -1,820 | |
| Be2O3 2– + 6H+ + 4e– = 2 Be + 3H2O | -1,387 | |
| BeO2 2– + 4H+ + 2e– = Be + 2H2O | -0,909 | |
|
Бор B | [BF4] – + 3e– = B + 4F – | -1,04 |
| H3BO3 + 3H+ + 3e– = B + 3H2O | -0,869 | |
| BO3 2– + 6H+ + 3e– = B + 3H2O | -0,165 | |
|
Бром Br | 2BrO– + 2H2O + 2e– = Br2 + 4OH – | +0,45 |
| BrO3– + 2H2O + 4e– = BrO + 4OH – | +0,54 | |
| BrO3– + 3H2O + 6e– = Br – + 6OH – | +0,61 | |
| BrO– + H2O + 2e– = Br2 + 4OH – | +0,76 | |
| Br3– + 2e– = 3Br – | +1,05 | |
| {Br2} + 2e– = 2Br – | +1,065 | |
| BrCl + 2e– = Br – + Cl – | +1,2 | |
| BrO3– + 6H+ + 6e– = Br – + 3H2O | +1,44 | |
| BrO3– + 5H+ + 4e– = HBrO + 2H2O | +1,49 | |
| 2BrO3– + 12H+ + 10e– = Br2 + 6H2O | +1,52 | |
| 2HBrO + 2H+ + 2e– = Br2 + 2H2O | +1,59 | |
|
Водород H | H2 + 2e– = 2H– | -2,251 |
| H+ + 2e– = H – | -1,125 | |
| 2H+ + 2e– = H2 | 0,000 | |
| H1 + H+ + e– = H2 | +2,106 | |
| 2H2O + 2e– = H2 + 2OH – | -0,828 | |
| Железо Fe | FeS + 2e– = Fe + S2– | -0,97 |
| FeCO3 + 2e– = Fe + CO32– | -0,756 | |
| Fe2S3– + 2e– = 2FeS + S2– | -0,7 | |
| Fe2+ + 2e– = Fe | -0,440 | |
| Fe3O4 + 8H+ + 8e– = 3Fe + 4H2O | -0,085 | |
| Fe2O3 + H2O + 2H+ + 2e– = 2Fe(OH)2 | -0,057 | |
| Fe2O3 + 6H+ + 6e– = 2Fe + 3H2O | -0,051 | |
| Fe(OH)2 + 2H+ + 2e– = Fe + 2H2O | -0,047 | |
| Fe(OH)3 + 3H+ + 3e– = Fe + 3H2O | +0,059 | |
| Fe(OH)3 + H+ + e– = Fe(OH)2 + H2O | +0,271 | |
| [Fe(CN)6]3 – + e– = [Fe(CN)6]4 – | +0,36 | |
| Fe3+ + e– = Fe2+ | +0,771 | |
| FeOH2+ + H+ + e– = Fe2+ + H2O | +0,914 | |
| Fe3O4 + 8H+ + 2e+ = 3Fe2+ + 4H2O | +0,980 | |
| FeOH42 – + 5H+ + 4e– = HFeO2 – + 2H2O | +1,001 | |
| FeO42 – + 8H+ + 3e– = Fe3+ + 4H2O | +1,700 | |
|
Золото Au | [Au(CN)2] – + e– = Au + 2CN | -0,60 |
| AuI + e– = Au + I – | +50 | |
| [Au(SCN)2] – + e– = Au + 2CSN – | +0,69 | |
| [AuBr4] – + 2e– = AuBr2 – + 2Br – | +0,82 | |
| AuO2 + H2O + e– = HAuO32 – + H+ | +0,822 | |
| [AuBr4]– + 2e– = Au + 4Br – | +0,87 | |
| [AuBr2]– + e– = Au + 2Br – | +0,96 | |
| [AuCl4]– + 3e– = Au + 4Cl – | +1,00 | |
| AuCl + e– = Au + Cl – | +1,17 | |
| Au3+ + 2e– = Au + | +1,401 | |
| Au2O3 + 6H+ + 6e– = 2Au + 3H2O | +1,457 | |
| Au3+ + 3e– = Au | +1,498 | |
| H3AuO3 + 3H+ + 2e– = Au+ + 3H2O | +1,502 | |
| AuO2 + 4H+ + e– = Au3+ + 2H2O | +2,507 | |
|
Иод I | IO3– + 3H2O + 6e– = I – + 6OH – | +0,26 |
| IO– + H2O + 2e– = I – + 2OH – | +0,49 | |
| I2 + 2e– = 2I – | +0,536 | |
| I3 + 3e– = 3I – | +0,536 | |
| IO3– + 2H2O + 4e– = IO – + 4OH – | +0,56 | |
| 2ICN + 2H+ + 2e– = I2 + 2HCN | +0,63 | |
| H3IO62 – + 2e– = IO3 – + 3OH – | +0,7 | |
| 2IBr2 + 4e– = I2 + 4Br – | +0,87 | |
| HIO + H+ + 2e– = I – + H2O | +0,99 | |
| 2IBr + 2e– = I2 + 2Br – | +1,02 | |
| 2ICl2 + 2e– = I2 + 4Cl – | +1,06 | |
| IO3– + 6H+ + 6e– = I – + 3H2O | +1,085 | |
| 2ICl + 2e– = I2 + 2Cl – | +1,19 | |
| 2IO3– + 12H+ + 10e– = I2 + 6H2O | +1,19 | |
| 2ICl2 + 6e– = I2 + 4Cl – | +1,28 | |
| 2HIO + 2H+ + 2e– = I2 + 2H2O | +1,45 | |
| H5IO6– + H+ + 2e– = IO3 – + 3H2O | +1,60 | |
| Калий K | K+ + e– = K | -2,924 |
| Кальций Са | Ca(OH)2 + 2e– = Ca + 2OH – | -3,03 |
| Ca2+ + 2e– = Ca | -2,866 | |
|
Кислород O | O2 + 2H+ + 2e– = O + H2O | +0,037 |
| O2 + H+ + 2e– = HO2 – | +0,338 | |
| O2 + 2H2O + 4e – = 4OH – | +0,401 | |
| O2 + 2H++ 2e– = H2O2 | +0,682 | |
| O2 + 4H+ + 4e– = 2H2O | +1,228 | |
| O3 + 6H+ + 6e– = 3H2O | +1,511 | |
| HO2– +2H+ + 2e– = H2O + OH– | +1,706 | |
| H2O2 + 2H+ + 2e– = 2H2O | +1,776 | |
|
| O3 + 2H+ + 2e– = O2 + H2O | +2,076 |
| O + 2H+ + 2e– = H2O | +2,421 | |
|
Кремний Si | SiF62 – + 4e– = Si + 6F – | -1,2 |
| SiO2 + 4H+ + 4e– = Si + 2H2O | -0,857 | |
| H2SiO3 + 4H+ + 4e– = Si + 3H2O | -0,807 | |
| SiO32 – + 6H+ + 4e– = Si + 3H2O | -0,455 | |
| Si + 4H+ + 4e– = SiH4 | +1,102 | |
| Литий Li | Li+ + e– = Li | -3,045 |
| Магний Mg | Mg2+ + 2e– = Mg | -2,363 |
| Mg(OH)2+ 2H+ + 2e– = Mg + 2H2O | -1,862 | |
|
Марганец Mn | MnCO3 + 2e– = Mn + CO32- | -1,48 |
| Mn2+ + 2e– = Mn | -1,179 | |
| Mn(OH)2 + 2H+ + 2e– = Mn + 2H2O | -0,727 | |
| [Mn(CN)6]3 – + e– = [Mn(CN)6]4 – | -0,22 | |
| MnO4 – + e– = Mn4O2 – | +0,564 | |
| MnO42 – + 2H2O + 3e– = MnO2 + 4OH – | +0,6 | |
| MnO2 + 4H+ + 2e– = Mn2+ + 2H2O | +1,228 | |
| Mn2O3 + 6H+ + 2e– = 2Mn2+ + 3H2O | +1,443 | |
| MnO4 – + 8H+ + 5e– = Mn2+ + 4H2O | +1,507 | |
| Mn3+ + e– = Mn2+ | +1,509 | |
| MnO4 – + 4H+ + 3e– = MnO2 + 2H2O | +1,692 | |
| MnO42– + 4H+ + 2e– = MnO2 + 2H2O | +2,257 | |
|
Медь Cu | Cu + H+ + e– = CuH | -2,775 |
| Cu2S + 2e– = 2Cu + S2– | -0,93 | |
| CuS + 2e– = Cu + S2– | -0,97 | |
| [Cu(CN)2]– + e– = Cu + 2CN – | -0,43 | |
| CuSCN + e– = Cu + SCN – | -0,27 | |
| CuI + e– = Cu + I – | -0,185 | |
| [Cu(NH3)2]+ + e– = Cu + 2NH3 | -0,12 | |
| [Cu(NH3)4]2+ + 2e– = Cu + 4NH3 | -0,05 | |
| CuI2– + e– = Cu + 2I– | +0,00 | |
| CuBr + e– = Cu + Br– | +0,03 | |
| CuCl + e– = Cu + Cl – | +0,137 | |
| Cu2+ + e– = Cu+ | +0,153 | |
| 2Cu2+ + H2O + 2e– = Cu2O + 2H+ | +0,203 | |
| Cu2+ + 2e– = Cu | +0,337 | |
| Cu2O + 2H+ + 2e– = 2Cu + H2O | +0,471 | |
| Cu+ + e– = Cu | +0,520 | |
| Cu2+ + Cl– + e– = CuCl | +0,538 | |
| CuO + 2H+ + 2e– = Cu + H2O | +0,570 | |
| Cu(OH)2 + 2H+ + 2e– = Cu + 2H2O | +0,609 | |
| Cu2+ + Br – + e– = CuBr | +0,640 | |
| 2CuO + 2H+ + 2e– = Cu2O + H2O | +0,669 | |
|
| Cu2+ + I– + e– = CuI | +0,86 |
| Cu2+ + CN– + e– = CuCN | +1,12 | |
| HCuO2– + 3H+ + 2e– = Cu + 2H2O | +1,127 | |
| CuO22– + 4H+ + 2e– = Cu + 2H2O | +1,515 | |
| Натрий Na | Na+ + e– = Na | +2,714 |
|
Никель Ni | g-NiS + 2e– = Ni + S2– | -0,99 |
| a-NiS + 2e– = Ni + S2– | -0,83 | |
| [Ni(CN)4]2– + e– = [Ni(CN)4]3– | -0,82 | |
| [Ni(CN3)6]2++ 2e– = Ni + 6NH3 | -0,49 | |
| NiCO3 + 2e– = Ni + CO32– | -0,45 | |
| Ni2+ + 2e– = Ni | -0,250 | |
| NI(OH)2 + 2H+ + 2e– = Ni + 2H2O | +0,110 | |
| NiO + 2H+ + 2e– = Ni + H2O | +0,116 | |
| HNiO2– + 3H+ + 2e– = Ni + 2H2O | +0,648 | |
| Ni3O4 + 2H+ + 2e– = 3NiO + H2O | +0,897 | |
| 3Ni2O4 + 2H+ + 2e– = 2Ni3O4 + H2O | +1,305 | |
| 2NiO2 + 2H+ + 2e– = 2Ni2O3 | +1,434 | |
| NiO2 + 4H+ + 2e– = Ni2+ + 2H2O | +1,593 | |
|
Олово Sn | Sn + 4H+ + 4e– = SnH4 | -1,074 |
| SnS + 2e– = Sn + S2– | -0,94 | |
| SnF62– + 4e– = Sn + 6F– | -0,25 | |
| Sn2+ + 2e– = Sn | -0,136 | |
| SnO2 + 2H+ + 2e– = SnO + H2O | -0,108 | |
| SnO2 + 4H+ + 4e– = Sn + 2H2O | -1,106 | |
| SnO + 2H+ + 2e– = Sn + H2O | -0,104 | |
| Sn(OH)2 + 2H+ + 2e– = Sn + 2H2O | -0,091 | |
| Sn(OH)4 + 4H+ + 4e– = Sn + 4H2O | -0,008 | |
| Sn4+ + 2e– = Sn2+ | +0,151 | |
| HSnO2– + 3H+ + 2e– = Sn + 2H2O | +0,333 | |
| SnO32– + 3H+ + 2e– = HSnO2– + H2O | +0,374 | |
|
Ртуть Hg | Hg + H+ + e– = HgH | -2,881 |
| HgS + 2e– = Hg + S2 – | -0,75 | |
| [Hg(CN)4]2– + 2e– = Hg + 4CN– | -0,37 | |
| Hg2I2 + 2e– = 2Hg + 2I– | -0,041 | |
| HgI4 + 4e– = Hg + 4I– | -0,04 | |
| Hg2Br2 + 2e– = 2Hg + 2Br– | +0,140 | |
| [HgBr4]2– + 2e– = 2Hg + 4Br – | +0,21 | |
| Hg2Cl2 + 2e– = 2Hg + 2Cl – | +0,267 | |
| [HgCl4]2– + 2e– = Hg + 4Cl – | +0,48 | |
| Hg2SO4 + 2e– = 2Hg + SO42– | +0,615 | |
| Hg22+ + 2e– = 2Hg | +0,850 | |
| Hg2+ + 2e– = Hg | +0,850 | |
| 2Hg2+ + 2e– = Hg22+ | +0,920 | |
|
| HgO + 2H+ + 2e– = Hg + H2O | +0,926 |
| Hg(OH)2 + 2H+ + 2e– = Hg + 2H2O | +1,034 | |
|
Свинец Pb | Pb + 2H+ + 2e– = PbH2 | -1,507 |
| PbS + 2e– = Pb + S2– | -0,98 | |
| PbCO3 + 2e– = Pb + CO32– | -0,506 | |
| PbI2 + 2e– = Pb + 2I– | -0,365 | |
| PbSO4 + 2e– = Pb + SO42– | -0,356 | |
| PbBr2 + 2e– = Pb + 2Br – | -0,280 | |
| PbCl2 + 2e– = Pb + 2Cl – | -0,268 | |
| Pb2+ + 2e– = Pb | -0,126 | |
| PbO + 2H+ + 2e– = Pb + H2O | +0,248 | |
| Pb(OH)2 + 2H+ + 2e– = Pb + 2H2O | +0,277 | |
| HPbO2– + 3H+ + 2e– = Pb + 2H2O | +0,702 | |
| Pb3O4 + 2H+ + 2e– = 3PbO + H2O | +0,972 | |
| 3PbO2 + 4H+ + 2e– = Pb3O4 + 2H2O | +1,127 | |
| PbO2 + 4H+ + 2e– = Pb2+ + 2H2O | +1,449 | |
| PbO32– + 3H+ + 2e– = HPbO2– + H2O | +1,547 | |
| PbO2 + SO42– + 4H+ + 2e– = PbSO4 + 2H2O | +1,685 | |
| Pb4+ + 2e– = Pb2+ | +1,694 | |
| PbO32– + 4H+ + 2e– = PbO + 2H2O | +2,001 | |
| 3PbO32– + 10H+ + 4e– = Pb3O4 + 5H2O | +2,515 | |
|
Серебро Ag | AgBrO3 + e– = Ag + BrO3– | +0,55 |
| AgNO2 + e– = Ag + NO2– | +0,562 | |
| AgCH3COO + e– = Ag + CH3COO – | +0,643 | |
| Ag+ + e– = Ag | +0,799 | |
| Ag2O + 2H+ + 2e– = 2Ag + H2O | +1,173 | |
| 2AgO + 2H+ + 2e– = Ag2O + H2O | +1,398 | |
| Ag2O3 + 2H+ + 2e– = 2AgO + H2O | +1,569 | |
| Ag2O3 + 6H+ + 4e– = 2Ag+ + 3H2O | +1,670 | |
| AgO+ + 2H+ + 2e– = Ag+ + H2O | +1,998 | |
| AgO– + 2H+ + e– = Ag + H2O | +2,220 | |
| Ag2S + 2e– = 2Ag + S22 – | -0,69 | |
| [Ag(CN)2]– + e– = Ag + 2CN – | -0,31 | |
| AgI + e– = Ag + I– | -0,152 | |
| AgCN + e– = Ag + CN– | -0,017 | |
| [Ag(S2O3)2]3– + e– = Ag + 2S2O32– | +0,01 | |
| AgBr + e– = Ag + Br– | +0,03 | |
| AgSCN + e– = Ag + SCN– | +0,09 | |
| Ag4[Fe(CN)6] + 4e– = 4Ag + [Fe(CN)6]4– | +0,194 | |
| AgCl + e– = Ag + Cl– | +0,222 | |
| AgN3 + e– = Ag + N3– | +0,292 | |
| AgIO3 + e– = Ag + IO3– | +0,35 | |
|
| [Ag(NH3)2]+ + e– = Ag + 2NH3 | +0,378 |
| AgSCN + e– = Ag + SCN– | +0,41 | |
| [Ag(SO3)2]3– + e– = Ag + 2SO32– | +0,43 | |
| Ag2CrO4 + 2e– = 2Ag + CrO42– | +0,446 | |
| Ag2C2O4 + e– = 2Ag + C2O42– | +0,472 | |
| Стронций Sr | Sr2+ + 2e– = Sr | -2,888 |
|
Сурьма Sb | SbS43– + 2e– = Sb2– + 2S2– | -0,6 |
| Sb + 3H+ + 3e– = SbH3 | -0,510 | |
| Sb2O3 + 6H+ + 6e– = 2Sb + 3H2O | +0,512 | |
| SbO+ + 2H+ + 3e– = Sb + H2O | +0,212 | |
| SbO3– + 2H+ + 2e– = SbO2– + H2O | +0,353 | |
| SbO2– + 4H+ + 3e– = Sb + 2H2O | +0,446 | |
| Sb2O5 + 4H+ + 4e– = Sb2O3 + 2H2O | +0,671 | |
| SbO2+ + 2H+ + 2e– = SbO+ + H2O | +0,720 | |
|
Cера S | SO42– + H2O + 2e– = SO32– + 2OH– | -0,93 |
| S22– + 2e– = 2S2– | -0,524 | |
| 2S32– + 2e– = 3S2– | -0,506 | |
| S + 2e– = S2– | -0,48 | |
| 3S42– + 2e– = 4S32– | -0,478 | |
| 2S + 2e– = S22– | -0,476 | |
| S + H+ + 2e– = HS– | -0,065 | |
| S2O32– + 6H+ + 8e– = 2S2– + 3H2O | -0,006 | |
| S52– + 5H+ + 8e– = 5HS– | +0,003 | |
| S2O32– + 2e– = 2SO32– | +0,026 | |
| S42– + 4H+ + 6e– = 4HS– | +0,033 | |
| S32– + 3H+ + 4e– = 3HS– | +0,097 | |
| SO42– + 8H+ + 8e– = S2– + 4H2O | +0,149 | |
| S + 2H+ + 2e– = H2S | +0,171 | |
| SO32– + 4H+ + 2e– = H2SO3 + H2O | +0,17 | |
| SO42– + 6H+ + 6e– = S2– + 3H2O | +0,231 | |
| HSO4– + 9H+ + 8e– = H2S + 4H2O | +0,289 | |
| S22– + 2H+ + 2e– = 2HS– | +0,298 | |
| SO42– + 10H+ + 8e– = H2S + 4H2O | +0,303 | |
| SO42– + 10H+ + 8e– = H2S + 4H2O | +0,311 | |
| SO42– + 8H+ + 6e– = S + 4H2O | +0,357 | |
| 2SO3 + 4H+ + 2e– = S2O42– + 2H2O | +0,416 | |
| H2SO3 + 4H+ + 4e– = S + 3H2O | +0,449 | |
| SO2 + 4H+ + 4e– = S + 2H2O | +0,451 | |
| 2SO32– + 6H+ + 4e– = S2O32– + 3H2O | +0,705 | |
| SO + 2H+ + 2e– = S + H2O | +1,507 | |
| S2O82– + 2e– = 2SO42– | +2,010 | |
| Углерод С | 2H2CO3 + 2H+ + 2e– = H2C2O4 + 2H2O | -0,386 |
| H2CO3 + 2H++ 2e– = HCO2H + H2O | -0,156 | |
|
| C + 4H+ + 4e– = CH4 | -0,132 |
| H2CO3 + 4H+ + 4e– = HCOH + 2H2O | -0,050 | |
| C2O42– + 2H+ + 2e– = 2HCO2– | +0,013 | |
| H2CO3 + 6H+ + 6e– = CH3OH + 2H2O | +0,044 | |
| HCO2H + 4H++ 4e– = CH3OH + H2O | +0,145 | |
| HCO2– + 3H+ + 2e– = HCOH + H2O | +0,167 | |
| CO32– + 6H+ + 4e– = HCOH + 2H2O | +0,197 | |
| H2CO2– + 5H+ + 4e– = CH3OH + H2O | +0,199 | |
| CO2 + 4H+ + 4e– = C + 2H2O | +0,207 | |
| CO32– + 8H+ + 6e– = CH3OH + 2H2O | +0,209 | |
| CO32– + 3H+ + 2e– = HCO2– + H2O | +0,227 | |
| H2CO32– + 4H+ + 4e– = C + 3H2O | +0,228 | |
| HCOOH + 2H+ + 2e– = CH3OH | +0,232 | |
| 2CO32– + 4H+ + 2e– = C2O42– + 2H2O | +0,441 | |
| CO32– + 6H+ + 4e– = C + 3H2O | +0,475 | |
| CO + 6H+ + 6e– = CH4 + H2O | +0,497 | |
| CO + 2H+ + 2e– = C + H2O | +0,518 | |
|
Фосфор P | H2PO2– + e– = P + 2OH– | -2,05 |
| HPO32– + 2H2O + 2e– = H2PO2– + 3OH– | -1,57 | |
| H2PO2– + 3H2O + 4e– = PH3 + 5OH– | -1,18 | |
| PO43– + 2H2O + 2e– = HPO32– + 3OH– | -1,12 | |
| 2H3PO4 + 2H+ + 2e– = H4P2O6 + 2H2O | -0,94 | |
| H3PO3 + 3H+ + 3e– = P + 3H2O | -0,502 | |
| H2PO4 + 5H+ + 5e– = P + 4H2O | -0,411 | |
| H3PO4 + 5H+ + 5e– = P + 4H2O | -0,383 | |
| H2PO4 + 2H+ + 2e– = H3PO3 + H2O | -0,276 | |
| P + 3H+ + 3e– = PH3 | +0,06 | |
| H4P2O6 + 2H+ + 2e– = 2H3PO3 | +0,38 | |
| Фтор F | F2O + 2H+ + 4e– = 2F– + H2O | +2,1 |
| F2 + 2e– = 2F– | +2,87 | |
| F2 + 2H+ + 2e– = 2HF | +3,06 | |
|
Хлор Cl | ClO3– + 3H2O + 6e– = Cl– + 6OH– | +0,63 |
| Cl2 + 2H+ + 2e– = 2HCl | +0,987 | |
| ClO4– + 2H+ + 2e– = ClO3– + H2O | +1,189 | |
| Cl2O + 4H+ + 4e– = 2HCl + H2O | +1,351 | |
| Cl2 + 2e– = 2Cl– | +1,359 | |
| 2ClO4– + 16H+ + 14e– = Cl2 + 8H2O | +1,385 | |
| ClO4– + 8H+ + 8e– = Cl– + 4H2O | +1,389 | |
| ClO2– + 5H+ + 5e– = HCl + 2H2O | +1,436 | |
| 2ClO3– + 12H+ + 10e– = Cl2 + 6H2O | +1,470 | |
| HClO + H+ + 2e– = Cl + H2O | +1,494 | |
| ClO2– + 4H+ + 5e– = Cl– + 2H2O | +1,511 | |
| 2ClO2 + 8H+ + 8e– = Cl2 + 4H2O | +1,540 | |
|
| 2ClO2 + 8H+ + 8e– = Cl2 + 4H2O | +1,549 |
| HClO2 + 3H+ + 4e– = Cl– + 2H2O | +1,570 | |
| 2HClO + 2H+ + 2e– = Cl2 + 2H2O | +1,594 | |
| 2HClO2 + 6H+ + 6e– = Cl2 + 4H2O | +1,628 | |
| 2HClO + 2H+ + 2e– = Cl2 + 2H2O | +1,630 | |
| 2HClO2 + 6H+ + 6e– = Cl2 + 4H2O | +1,640 | |
| Cl2O + 2H+ + 2e– = Cl2 + H2O | +1,879 | |
| Cl2O + 2H+ + 2e– = Cl2 + H2O | +1,714 | |
| Cl2O + 2H+ + 4e– = 2Cl– + H2O | +2,152 | |
|
Хром Cr | Cr2+ + 2e– = Cr | -0,913 |
| Cr3+ + 3e– = Cr | -0,744 | |
| Cr(OH)3 + 3H+ + 3e– = Cr + 3H2O | -0,654 | |
| CrO + 2H+ + 2e– = Cr + H2O | -0,588 | |
| Cr3+ + e– = Cr2+ | -0,407 | |
| CrO2– + 4H+ + 3e– = Cr + 2H2O | +0,213 | |
| Cr2O7– + 14H+ + 12e– = 2Cr3+ + 7H2O | +0,294 | |
| H2CrO4 + 6H+ + 6e– = Cr + 4H2O | +0,295 | |
| CrO42– + 2H+ + 3e– = CrO33– + H2O | +0,359 | |
| CrO42– + 8H+ + 6e– = Cr + 4H2O | +0,366 | |
| CrO33– + 6H+ + 3e– = Cr + 3H2O | +0,374 | |
| CrO42– + 4H+ + 3e– = CrO2– + 2H2O | +0,945 | |
| CrO2– + 4H+ + e– = Cr2+ + 2H2O | +1,188 | |
| Cr2O72– + 14H+ + 6e– = 2Cr3+ + 7H2O | +1,333 | |
| H2CrO4 + 6H+ + 3e– = Cr3+ + 4H2O | +1,335 | |
| CrO42– + 4H+ + 2e– = CrO2 + 2H2O | +1,437 | |
| CrO42– + 8H+ + 3e– = Cr3+ + 4H2O | +1,477 | |
| CrO2 + 4H+ + e– = Cr3+ + 2H2O | +1,556 | |
|
Цинк Zn | ZnS + 2e– = Zn + S2– | -1,44 |
| [Zn(CN)4]2– + 2e– = Zn + 4CN– | -1,26 | |
| ZnCO3 + 2e– = Zn + CO32– | -1,06 | |
| [Zn(NH3)4]2+ + 2e– = Zn + 4NH3 | -1,04 | |
| Zn2+ + 2e– = Zn | -0,763 | |
| Zn(OH)2 + 2H+ + 2e– = Zn– + 2H2O | -0,439 | |
| HZnO2– + 3H+ + 2e– = Zn + 2H2O | +0,054 | |
| ZnO22– + 4H+ + 2e– = Zn + 2H2O | +0,441 |
Таблица 18. «Электрическое сопротивление химических элементов,
10-8 Ом ´ м, (при 298 К)».
| Номер элемента | Элемент | Электрическое сопротивление | Номер элемента | Элемент | Электрическое сопротивление |
| 1 | Н | Данных нет | 54 | Xe | Данных нет |
| 2 | He | Данных нет | 55 | Cs | 20,0 |
| 3 | Li | 8, 55 | 56 | Ba | 50 |
| 4 | Be | 4,0 | 57 | La | 57 |
| 5 | B | 1,81012 | 58 | Ce | 73 |
| 6 | C(графит) | 1,375103 | 59 | Pr | 68 |
| 7 | N | Данных нет | 60 | Nd | 64,0 |
| 8 | O | Данных нет | 61 | Pm | 50(оценка) |
| 9 | F | Данных нет | 62 | Sm | 88,0 |
| 10 | Ne | Данных нет | 63 | Eu | 90,0 |
| 11 | Na | 4,2 | 64 | Gd | 134,0 |
| 12 | Mg | 4,45 | 65 | Tb | 114 |
| 13 | Al | 2,6548 | 66 | Dy | 57,0 |
| 14 | Si | 1,105 | 67 | Ho | 87,0 |
| 15 | P (Р4) | 1,1017 | 68 | Er | 87 |
| 16 | S | 2,1023 | 69 | Tm | 79,0 |
| 17 | Cl | Данных нет | 70 | Yb | 29,0 |
| 18 | Ar | Данных нет | 71 | Lu | 79,0 |
| 19 | K | 6,15 | 72 | Hf | 35,1 |
| 20 | Ca | 3,43 | 73 | Ta | 12,45 |
| 21 | Sc | 61,0 | 74 | W | 5,65 |
| 22 | Ti | 42,0 | 75 | Re | 19,3 |
| 23 | V | 24,8 | 76 | Os | 8,12 |
| 24 | Cr | 12,7 | 77 | Ir | 5,3 |
| 25 | Mn | 185,0 | 78 | Pt | 10,6 |
| 26 | Fe | 9,71 | 79 | Au | 2,35 |
| 27 | Co | 6,24 | 80 | Hg | 94,1 |
| 28 | Ni | 6,84 | 81 | Tl | 18,0 |
| 29 | Cu | 1,6730 | 82 | Pb | 20,648 |
| 30 | Zn | 5,916 | 83 | Bi | 106,8 |
| 31 | Ga | 27 | 84 | Po | 140 |
| 32 | Ge | 4,6105 | 85 | At | Данных нет |
| 33 | As | 26 | 86 | Rn | Данных нет |
| 34 | Se | 1,106 | 87 | Fr | Данных нет |
| 35 | Br | Данных нет | 88 | Ra | 100 |
| 36 | Kr | Данных нет | 89 | Ac | Данных нет |
| 37 | Rb | 12,5 | 90 | Th | 13,0 |
| 38 | Sr | 23,0 | 91 | Pa | 17,7 |
| 39 | Y | 57,0 | 92 | U | 30,8 |
| 40 | Zr | 40,0 | 93 | Np | 122 |
| 41 | Nb | 12,5 | 94 | Pu | 146 |
| 42 | Mo | 5,2 | 95 | Am | 68 |
| 43 | Tc | 22,6(373 К) | 96 | Cm | Данных нет |
| 44 | Ru | 7,6 | 97 | Bk | Данных нет |
| 45 | Rh | 4,51 | 98 | Cf | Данных нет |
| 46 | Pd | 10,8 | 99 | Es | Данных нет |
| 47 | Ag | 1,59 | 100 | Fm | Данных нет |
| 48 | Cd | 6,83 | 101 | Md | Данных нет |
| 49 | In | 8,37 | 102 | No | Данных нет |
| 50 | Sn(a) | 11,0 | 103 | Lr | Данных нет |
| 51 | Sb | 39 | 104 | Rf | Данных нет |
| 52 | Te | 4,36105 | 105 | Ha | Данных нет |
| 53 | I | 1,31015 | 106 | Unh | Данных нет |
Данные по электрическому сопротивлению вновь открытых элементов - № № 107 Ns, 108 Hs, 109 Mt, 110 Ds - отсутствуют.
Таблица 19. «Энтальпии образования в газовой фазе ∆Нообразования кДж/моль (298 К; 0,1 МПа)».
| Номер элемента | Элемент | Энтальпия образования | Номер элемента | Элемент | Энтальпия образования |
| 1 | Н | 218,0 | 54 | Xe | 0 |
| 2 | He | 0 | 55 | Cs | 76,1 |
| 3 | Li | 159,4 | 56 | Ba | 180 |
| 4 | Be | 324,3 | 57 | La | 431,0 |
| 5 | B | 562,7 | 58 | Ce | 423 |
| 6 | C | 716,7 | 59 | Pr | 355,6 |
| 7 | N | 472,7 | 60 | Nd | 327.6 |
| 8 | O | 249,2 | 61 | Pm | Данных нет |
| 9 | F | 79,0 | 62 | Sm | 206.7 |
| 10 | Ne | 0 | 63 | Eu | 175.3 |
| 11 | Na | 107,3 | 64 | Gd | 397.5 |
| 12 | Mg | 147,7 | 65 | Tb | 388.7 |
| 13 | Al | 326,4 | 66 | Dy | 290.4 |
| 14 | Si | 455,6 | 67 | Ho | 300,8 |
| 15 | P | 314,6 | 68 | Er | 317,1 |
| 16 | S | 278,8 | 69 | Tm | 232,2 |
| 17 | Cl | 121,7 | 70 | Yb | 152,3 |
| 18 | Ar | 0 | 71 | Lu | 427,6 |
| 19 | K | 89,2 | 72 | Hf | 619,2 |
| 20 | Ca | 178,2 | 73 | Ta | 782,0 |
| 21 | Sc | 377,8 | 74 | W | 849,4 |
| 22 | Ti | 469,9 | 75 | Re | 769,9 |
| 23 | V | 514,2 | 76 | Os | 791 |
| 24 | Cr | 396,6 | 77 | Ir | 665,3 |
| 25 | Mn | 280,7 | 78 | Pt | 565,3 |
| 26 | Fe | 416,3 | 79 | Au | 366,1 |
| 27 | Co | 424,7 | 80 | Hg | 61,3 |
| 28 | Ni | 429,7 | 81 | Tl | 182,2 |
| 29 | Cu | 338,3 | 82 | Pb | 195,0 |
| 30 | Zn | 130,7 | 83 | Bi | 207,1 |
| 31 | Ga | 277,0 | 84 | Po | 146 |
| 32 | Ge | 376,6 | 85 | At | Данных нет |
| 33 | As | 302,5 | 86 | Rn | 0 |
| 34 | Se | 227,1 | 87 | Fr | 72,8 |
| 35 | Br | 111,9 | 88 | Ra | 159 |
| 36 | Kr | 0 | 89 | Ac | 406 |
| 37 | Rb | 80,9 | 90 | Th | 598,3 |
| 38 | Sr | 164,4 | 91 | Pa | 607 |
| 39 | Y | 421,3 | 92 | U | 535,6 |
| 40 | Zr | 608,8 | 93 | Np | Данных нет |
| 41 | Nb | 725,9 | 94 | Pu | Данных нет |
| 42 | Mo | 658,1 | 95 | Am | Данных нет |
| 43 | Tc | 642,7 | 96 | Cm | Данных нет |
| 44 | Ru | 642,7 | 97 | Bk | Данных нет |
| 45 | Rh | 556,9 | 98 | Cf | Данных нет |
| 46 | Pd | 378,2 | 99 | Es | Данных нет |
| 47 | Ag | 284,6 | 100 | Fm | Данных нет |
| 48 | Cd | 112,0 | 101 | Md | Данных нет |
| 49 | In | 243,3 | 102 | No | Данных нет |
| 50 | Sn | 302,1 | 103 | Lr | Данных нет |
| 51 | Sb | 262,3 | 104 | Rf | Данных нет |
| 52 | Te | 196,7 | 105 | Ha | Данных нет |
| 53 | I | 106,8 | 106 | Unh | Данных нет |
Данные по энтальпии образования в газовой фазе вновь открытых элементов - № № 106 Unh, 107 Ns, 108 Hs, 109 Mt, 110 Ds - отсутствуют.
Таблица 20. «Энтальпии испарения ∆Ниспарения , кДж/моль».
| Номер элемента | Элемент | Энтальпия образования | Номер элемента | Элемент | Энтальпия образования |
| 1 | Н | 0,46 | 54 | Xe | 12,65 |
| 2 | He | 0,082 | 55 | Cs | 66,5 |
| 3 | Li | 147,7 | 56 | Ba | 150,9 |
| 4 | Be | 308,8 | 57 | La | 402,1 |
| 5 | B | 504,5 | 58 | Ce | 398 |
| 6 | C | 710,9 | 59 | Pr | 357 |
| 7 | N | 5,58 | 60 | Nd | 328 |
| 8 | O | 6,82 | 61 | Pm | Данных нет |
| 9 | F | 3,26 | 62 | Sm | 164,8 |
| 10 | Ne | 1,736 | 63
 Сейчас читают про:
|
 2018-02-14
2018-02-14 199
199