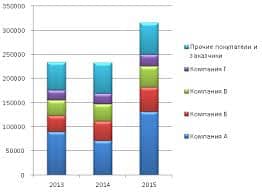
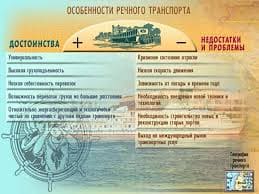
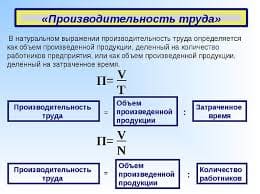
Exercise 1. Describe electrochemical effect; battery; current; solution
using the suggested words and expressionsas in example:
| electrochemical effect conversion; electric energy; electrochemical cells; reverse process; elemental aluminum; magnesium; bromine; compounds; elements. example:The electrochemical effect is a conversion of chemical to electric energy, as in electrochemical cells; or the reverse process, used to produce elemental aluminum, magnesium, and bromine from compounds of these elements. |
| battery direct-current voltage; source; to convert; chemical, thermal, nuclear, or solar energy; electrical energy. |
| current transfer; electric charge; device; a circuit; electric current. |
| solution homogeneous liquid; solid, or gas phase; a mixture; components (liquid, gas, solid, or combinations thereof); uniformly; to distribute. |
Exercise 2. Ask questions to the given answers:
1) Question: ___________________________________________?
Answer: Battery energy is used to operate the lighting and accessory systems.
2) Question: ___________________________________________?
Answer: The chemical reaction totally destroys one of the metals after a period of time.
3) Question: ___________________________________________?
Answer: The battery is built, charged, washed and dried, sealed, and shipped without electrolyte.
4) Question: ___________________________________________?
Answer: Today’s batteries are more durable, have high power-to-weight ratios and lighter cases.
5) Question: ___________________________________________?
Answer: The acid is a mixture of 25 percent water and 75 percent sulfuric acid (H2SO4).

III. Writing exercises:
Exercise 1. Complete the sentences with the suggested words: series;output; which; discharges; greater
A 12-volt battery has six “cells,” each of _______ contains 9 to 20 positive and negative plates. The ________ the number of plates, the higher the power output (measured in amps) of the battery. Each cell produces 2.11 volts, so when all six cells are connected together in______, the battery’s total ______ is actually 12.66 volts. As a battery ________, sulfate combines chemically with the positive and negative plates.
Exercise 2. Fill in the table with words and expressions from the text:
| parts | material | processes | |
| Example: lead in an acid mixture react to produce | an electrical pressure. | ||
| the battery also serves as | |||
| a slow chemical reaction will cause | |||
| sulfate combines chemically with |
Exercise 3. Compose a story on one of the topics (up to 100 words):
“An automotive lead-acid battery”
“The lead-acid battery”
“ Battery energy”
“Electrical energy on vehicles”
Lesson 5

Read the text: NEW CELL DESIGNS
Many batteries today contain a "gel" electrolyte. There is no free liquid inside the battery. The acid is contained in Absorbent Glass Mat (AGM) separators between the plates. The separators hold the acid much like a paper towel soaks up water, making the battery spill-proof even if the case is punctured. This also means a battery with AGM separators can be shipped by normal means (no spillage) and installed in any position. AGM separators provide extra cushioning and are more resist to vibration damage (a leading cause of battery failure).
Some premium batteries also use a new type of "spiral-wound" cell construction instead of flat plates. This allows a greater surface area for higher power density. The six battery cells are arranged like beverage cans in a six-pack. The result is a very compact, efficient design that delivers more cranking amps during the initial three to five seconds of cranking. The spiral-wound cells also produce a slightly higher voltage that raises the battery's overall voltage output by 15 percent for added cranking power. The tightly wound spiral cells typically hold a charge longer than flat plate cells, which means the battery can sit for months without being used and still crank an engine. The spiral-wound construction also offers improved durability making this type of battery ideal for rugged-use applications.
New high-voltage batteries (up to 274 volts) are also being used in hybrid vehicles. In the first-generation Toyota Prius, a stack of 38 7.2 volt Nickel Metal Hydride (NHM) batteries are combined in series to produce the power needed to run the car’s electric motor and operate the engine stop-start system. The second generation Prius uses a smaller 28 NHM battery pack with a voltage rating of only 201 volts, but it’s amperage output is actually higher than the previous battery. These are very expensive batteries and are covered by an extended warranty. Hybrid replacement batteries are not yet available in the aftermarket - but that will eventually change as more and more hybrid vehicles go into production. Both Ford and GM have hybrids coming (though in limited numbers), and Toyota and Honda are ramping up their production.
 2018-02-14
2018-02-14 287
287