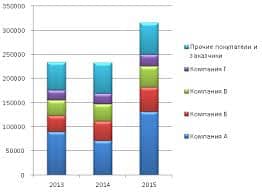
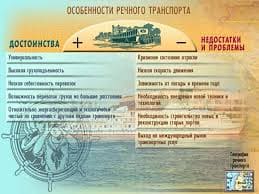
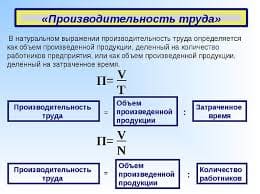
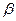 кварц широко распространен в природе как составная часть горных пород. В природе
кварц широко распространен в природе как составная часть горных пород. В природе 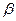 кварц встречается в виде горного хрусталя (прозрачная разновидность кварца), жильного кварца, кварцевых песков, халцедона, агата, яшмы, кремния.
кварц встречается в виде горного хрусталя (прозрачная разновидность кварца), жильного кварца, кварцевых песков, халцедона, агата, яшмы, кремния.
Природные разновидности 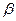 кварца применяются при изготовлении оптических и электрических приборов, в ювелирном деле, производстве тонкой и грубой керамики.
кварца применяются при изготовлении оптических и электрических приборов, в ювелирном деле, производстве тонкой и грубой керамики.
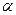 кварц близок по структуре
кварц близок по структуре 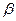 кварцу. В природных условиях не встречается.
кварцу. В природных условиях не встречается.
Тридимит содержится в кислых породах. Кристабалит найден в стеклах, горных породах.
Lecture 17 ФХС
Theme: the phenomenon полиморфизма and изоморфизма. Изоморфные of replacement in силикатах. Firm solutions in силикатах. The diagram SiO2. Проследовательность of phase transformations and characteristic of the polymorphic forms.
At research of equilibrium systems a ultimate goal of the физико-chemical analysis is the establishment of dependence between parameters of system describing her(it), condition and in particular definition of structures of equilibrium phases at those or other parameters of a condition.
The thermodynamic diagram represents the diagram on which on an axis of coordinates postpone meanings(importance) of thermodynamic parameters or functions of a condition. Such diagrams allow to describe change of properties of system at change of its(her) parameters.
The feature of the diagrams of a condition consists that any point on the diagram has strict физико-chemical sense. In other words each condition of system is represented on the diagram by some point, which is called as a figurative point.
The diagrams of a condition have the extremely important meaning(importance) for many areas of an industry, in particular of microelectronics, chemical technology, technologies силикатных and refractory not metal materials.
These diagrams allow to decide(solve) a number(line) both important practical and theoretical problems of the various substances, connected to reception, with a various combination of properties.
The diagram of a condition in many cases enables to explain and to improve processes proceeding at education of the given material, to establish and to explain influence of phase structure of a product on his(its) properties for reception of a material beforehand by given properties.
It is necessary to note, that is direct in process обжига many силикатных of products at high temperatures, when the liquid phase of their condition is formed comes nearer to equilibrium. This balance is broken usually only in process of too fast cooling, which takes place at manufacturing many силикатных of products.
Полиморфизмом the ability of substance of the same structure is called to exist depending on external conditions in the several crystal forms (polymorphic updatings) with various structure (for simple substances this phenomenon sometimes name аллотропией).
To external conditions determining полиморфизм, concern temperature and pressure.
As firm solutions of replacement such solutions are called which are formed as a result of static replacement of atoms or ions in structure any of crystal substance (solvent or matrix) atoms or ions of other (dissolved) substance borrowing(occupying) as a result of it regular units of a crystal lattice.
The diagram SiO2. Проследовательность of phase transformations and characteristic of the polymorphic forms.
Однокомпонентная the system SiO2 is system with complex(difficult) полиморфизмом. Is totaled 10 crystal and two стеклообразные of the form SiO2.
The transformation of updatings SiO2 submits to the following circuit (on Феннеру):
870 1470 1728


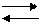
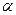 кварц
кварц 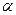 тридимит
тридимит 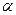 кристаболит расплав
кристаболит расплав
 575
575
167 230
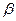 кварц
кварц 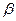 тридимит
тридимит 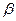 кристаболит
кристаболит
120
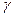 тридимит
тридимит
The main updatings SiO2 is the quartz, тридимит, кристаболит. Модификационные the transformations of crystal versions кремнезема can be divided(shared) into two groups: quickly proceeding and slowly proceeding.
The transformations of updatings concern to the first group within the limits of 1 mineral version:
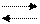
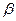 кварц
кварц 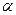 кварц;
кварц;
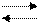

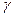 тридимит
тридимит 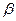 тридимит
тридимит 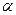 тридимит;
тридимит;

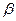 кристаболит
кристаболит 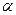 кристаболит
кристаболит
These transformations are accompanied by small changes in lattices кремнезема.
The transformations from one mineral version in another concern to the second group:


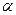 кварц
кварц 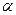 тридимит
тридимит 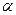 кристаболит
кристаболит
These transformations are accompanied by deep reorganization of a lattice. Steady updatings in this system are four firm phases:
Quartz, quartz, тридимит, кристаболит.
She a liquid phase - расплав, one газообразная a phase - pairs.
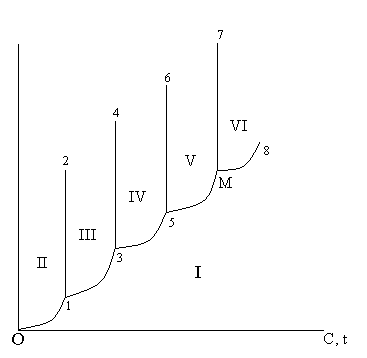
The line 0 - 1 - 3 - 5 -М - 8 divides a plane between coordinate axes into two parts.
In I parts all points display such conditions: temperature, pressure, at which SiO2 is as pair.
Area II a field of quartz.
Area III a field of quartz.
Area IV a field тридимит.
Area VI a field расплава.
Thus the line 1- 2 represents a condition of an equilibrium condition between quartz and quartz, and line 3 - 4 between quartz and тридимитом.
The threefold points 1 - 3 - 5 characterize an equilibrium condition of two firm phases and pair SiO2.
Point М - equilibrium condition кристаболита расплава SiO2 and pair SiO2.
The transformation of quartz in тридимит and тридимит in кристаболит is made slowly and complete end needs duration of time. Therefore at fast transformation of quartz he is higher 870 0С has not time to proceed(pass) in тридимит and кристаболит and there can be some time in updating quartz in unstable or метастабильном condition down to 1600 С. above than this temperature the quartz turns in расплав.
If расплав SiO2 to cool quickly, he has not time закристаллизоваться and stiffens as стеклообразной of weight (quartz sand)
In all these кодификационных transformations density changes. The general(common) increase of density makes 16,5 %. It has the large meaning(importance) in manufacture. Except for these updatings кремнезема 4 new updatings are open: коэсит, китит, стишовит, fibrous SiO2. all polymorphic updatings SiO2 differ from each other by optical properties of density, factor of linear expansion, structure of a crystal lattice and others.
Polymorphic updatings SiO2.
The quartz is widely distributed in a nature as a component of mountain breeds. In a nature the quartz meets as mountain crystal (transparent version of quartz), жильного of quartz, quartz sand, халцедона, агата, яшмы, silicon.
The natural versions of quartz are applied at manufacturing optical and electrical devices, in jeweller business, manufacture of thin and rough ceramics.
The quartz is close on structure to quartz. Naturally does not meet.
Тридимит contains in sour breeds. Кристабалит is found in glasses, mountain breeds.
 2014-02-17
2014-02-17 2411
2411