Figure shows the relative enhancements of NOx, CO, O3, and OH computed with the same model from preindustrial times to today. The preindustrial simulation assumes no emission from fossil fuel combustion and a much reduced emission from biomass burning. Results suggest that anthropogenic emissions have increased NOx and CO concentrations in most of the troposphere by factors of 2-8 (NOx) and 3-4 (CO). Ozone concentrations have increased by 50-100% in most of the troposphere, the largest increases being at low altitudes in the northern hemisphere.
The anthropogenic influence on OH is more complicated. Increasing NOx and O3 act to increase OH, while increasing CO and hydrocarbons act to deplete OH (section 11.3). Because CO and CH4 have longer lifetimes than NOx and O3, their anthropogenic enhancements are more evenly distributed in the troposphere. It is thus found in the model that the net effect of human activity is to increase OH in most of the lower troposphere and to decrease OH in the upper troposphere and in the remote southern hemisphere. There is compensation on the global scale so that the global mean OH concentration decreases by only 7% since preindustrial times (other models find decreases in the range 5-20%). The relative constancy of OH since preindustrial times is remarkable in view of the several-fold increases of NOx, CO, and CH4. There remain large uncertainties in these model analyses. From the CH3CCl3 observational record, which started in 1978, we do know that there has been no significant global change in OH concentrations for the past 20 years.
Формирование и разрушение озона в условиях загрязненной атмосферы
The high concentrations of O3 in surface air over the United States arise from high emissions of NOx and of various reactive hydrocarbons (including alkanes, alkenes, aromatics, and multifunctional compounds). The emission of NOx is mainly from fossil fuel combustion. The hydrocarbons are emitted by a range of human activities including combustion, fuel evaporation, solvent use, and chemical manufacturing. They also have a large natural source from terrestrial vegetation (the smell of a pine forest, for example, is due to natural hydrocarbons). Production of O3 in polluted air follows the same chain reaction mechanism as described in tropospheric chemistry. The chain is initiated by production of HOx,
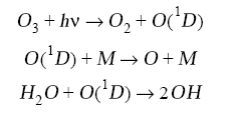
and is propagated by reaction of OH with hydrocarbons. We use RH (where R is an organic group) as simplified notation for hydrocarbons. Oxidation of a hydrocarbon by OH produces an organic peroxy radical RO2:
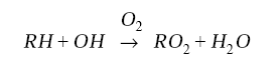 (4)
(4)
The relative importance of different hydrocarbons in driving this reaction can be measured in terms of their abundance and their reactivity with OH. The reactivity generally increases with the size of the hydrocarbon because of the larger number of C-H bonds available for H abstraction by OH; unsaturated hydrocarbons are also highly reactive because OH adds rapidly to the C=C double bonds. In surface air over the United States, large alkanes and unsaturated hydrocarbons are sufficiently abundant to dominate over CO or CH4 as sinks of OH, in contrast to the remote troposphere. The RO2 radical produced in this reactionreacts with NO to produce NO2 and an organic oxy radical RO:
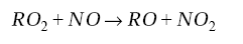 (5)
(5)
NO2 goes on to photolyze and produce O3. The RO radical has several possible fates. It may react with O2, thermally decompose, or isomerize. The subsequent chemistry is complicated. Typically, carbonyl compounds and a HO2 radical are produced. A generic representation of the reaction, following the fate of CH3O is
(6)
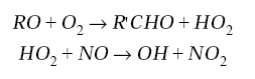 (7)
(7)
The carbonyl compound R’CHO may either photolyze to produce HOx (branching the chain, as we saw for CH2O) or react with OH to continue the chain propagation. The net reaction is
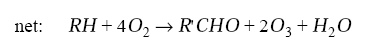
The chain is terminated by loss of HOx radicals. This loss takes place in two principal ways. When NOx concentrations are not too high, peroxy radicals may react with themselves instead of with NO to produce peroxides and other oxygenated compounds. The most important process is the self-reaction of HO2, as in the remote troposphere:
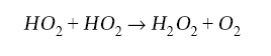 (8)
(8)
At very high NOx concentrations, the dominant sink for HOx radicals is the oxidation of NO2 by OH:
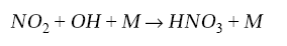 (9)
(9)
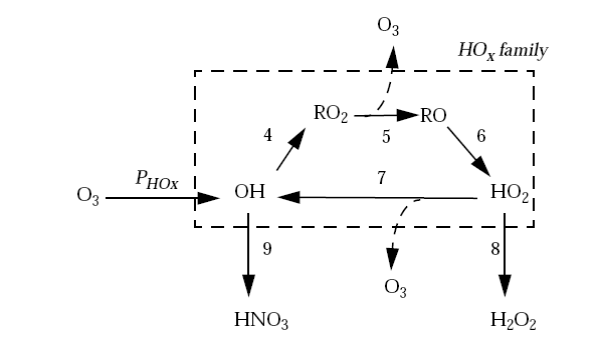
Figure is a diagram of the mechanism. The organic radicals RO2 and RO propagate the reaction chain and are thus considered part of the HOx radical family. One chain propagation cycle produces two O3 molecules (one from reaction (R5) and one from reaction (R7)).
 2014-02-09
2014-02-09 406
406






