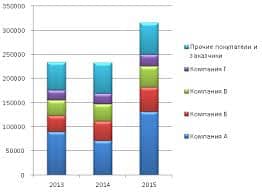
Эксперт:
Полученная дополнительная информация о повторных эпизодах потемнения мочи в анамнезе, а также наличие скоплений железа в клетках канальцев свидетельствует о хроническом, рецидивирующем течении гемолиза, что характерно для PNH. Результаты исследования биоптата почки указывают на развитие острого тубулярного некроза с формированием гемоглобиновых цилиндров, причиной которого стала гемоглобинурия. У пациентов с длительно текущей PNH обнаруживалось, в той или иной степени, накопление гемосидерина в проксимальных канальцах. У пациентов с этим заболеванием также может развиваться в умеренной степени хроническая почечная недостаточность и нарушение концентрационной способности почек. Следует получить подтверждение диагноза с помощью тестов на общий дефицит гликозилфосфатидилинозитоловых (GPI) “якорей” (в оригинале – glycosylphosphatidylinositol anchors; прим. перев.) или GPI-связанных поверхностных протеинов, таких как CD55 или CD59.
Пациентке выполнен анализ периферической крови с помощью поточной цитометрии, выявлен пониженный уровень GPI-связанного белка CD59 на эритроцитах, моноцитах и гранулоцитах, что позволило поставить диагноз PNH. На протяжении дальнейшего пребывания в стационаре у пациентки не возникало новых эпизодов гемолиза, сывороточный уровень креатинина постепенно снизился до 3,0 мг/дл (270 мкмоль/л), и она была выписана в удовлетворительном состоянии. Пациентке был назначен прием препаратов железо-полисахаридного комплекса дважды в день. Месяц спустя она посетила своего лечащего врача, в это время уровень креатинина составлял 1,1 мг/дл (97 мкмоль/л), гематокрит – 36,8%.
Комментарии.
Врачи часто сталкиваются с такой ситуацией, когда у находящихся в стационаре пациентов возникает новая патология, не связанная с изначальной причиной госпитализации. У данной пациентки, получающей лечение антикоагулянтами по поводу фибрилляции предсердий, развилась гемоглобинурия и почечная недостаточность. Первыми симптомами было снижение гематокрита и изменение окраски мочи на темно-красную, поэтому вначале можно было заподозрить, что это состояние связано с антикоагулянтной терапией. Однако последующее исследование мочи показало, что у пациентки скорее гемоглобинурия, чем гематурия; эти данные, в сочетании с наличием почечной недостаточности, подтолкнули к рассмотрению такого диагноза, как пигмент-индуцированная нефропатия. Такая форма повреждения почек чаще всего наблюдается при обширных раздавливаниях мягких тканей или рабдомиолизе, когда в роли нефротоксического агента выступает миоглобин.
Так как молекулы гемоглобина больше, чем миоглобина, и, к тому же, связаны с гаптоглобином, гемоглобин хуже фильтруется через почечные клубочки, поэтому его содержание в сыворотке для развития нефротоксического эффекта должно быть значительно выше по сравнению с миоглобином. Массивная гемоглобинурия встречается в случаях переливания несоответствующей крови, кроме того, есть отдельные сообщения о развитии гемоглобинурии, связанной с приемом различных медикаментов, в частности триамтерена, рифампицина и метилдофы. К другим причинам гемоглобинурии, которые также могут приводить к почечной недостаточности, относятся инфекции (брюшной тиф, малярия, эпидемический паротит и клостридиальный сепсис), укусы змей и скорпионов, тяжелые физические нагрузки (маршевая гемоглобинурия) и PNH. Если бы анамнестические данные о предшествовавших эпизодах гемоглобинурии у этой пациентки были бы получены раньше, то диагноз PNH мог быть поставлен гораздо быстрее.
PNH относится к редким заболеваниям (по расчетам, распространенность составляет 4-6:1.000.000 населения), характеризуется повторными приступами гемоглобинурии, вызванной внутрисосудистым гемолизом. Чаще моча описывается как имеющая цвет “чая” или “колы”, но почти у трети пациентов она может быть ярко-красной. Обычно моча имеет наиболее темную окраску утром, и в течение дня становится более прозрачной, от этого произошло определение “ночная” в названии заболевания. Пароксизмальная природа заболевания связана с интерметтирующим комплемент-зависимым разрушением эритроцитов, которое может провоцироваться инфекцией, хирургическими вмешательствами, гемотрансфузиями или вакцинацией. Однако, в большинстве случаев, причину обострения PNH установить не удается. Предыдущие эпизоды изменения цвета мочи у данной пациентки были по времени связаны с перенесенными вирусными инфекциями и могли быть именно таким образом связаны с активацией комплемента.
Причиной PNH является мутация в гене PIG-A, находящемся в X хромосоме. Этот ген кодирует белок, необходимый для формирования GPI-якорей на поверхности разнообразных клеток крови. При этом заболевании клетки крови лишены нескольких поверхностных белков, из которых лучше всего изучены факторы защиты от комплемента CD55 и CD59, в норме подавляющие связывание C9 с мембраной (имеется в виду компонент комплемента С9; прим. перев.). Отсутствие этих поверхностных белков на эритроцитах приводит, в условиях активации каскада комплемента, к развитию гемолиза. Относительно новый метод диагностики PNH основан на использовании меченного флюоресцентом инактивированноого белка аэролизина, который селективно связывается с GPI-якорями, позволяя определить отсутствие GPI-якорей на клетках крови. Поражается только часть клеток (известная как патологический клон); чем большая доля клеток изменена, тем тяжелее гемолиз и выше риск тромботических осложнений.
При внутрисосудистом гемолизе происходит выброс в плазму крови свободного гемоглобина, который в этом случае выступает в качестве мощного поглотителя оксида азота (NO) и может вызвать резкое снижение уровня NO в плазме. Снижение плазменного уровня оксида азота может быть непосредственной причиной многих симптомов PNH, в том числе болей в животе, спазмов пищевода, эректильной дисфункции и венозных тромбозов. Тромбозы обычно возникают в венозной системе брюшной полости и могут приводить к развитию кишечной непроходимости, ишемического колита, гастроинтестинального кровотечения или обструкции печеночных вен. У пациентов с PNH может развиваться гипоплазия костного мозга с подавлением эритроидного и миелоидного ростков, до 13% случаев приводят к апластической анемии.
Имеются многочисленные сообщения о случаях развития у пациентов с PNH острой почечной недостаточности, иногда настолько тяжелой, что потребовалось временное назначение гемодиализа. Также сообщается, как и в данном случае, о развитии у пациентов с PNH протеинурии различной степени выраженности, связанной, как предполагается, с поражением канальцев. Почечная недостаточность встречается приблизительно у 5% пациентов, страдающих PNH.
Как правило, у пациентов с PNH развивается дефицит железа, и они нуждаются в пероральном приеме препаратов железа. В некоторых случаях, прием этих препаратов может вначале вызывать приступ гемоглобинурии, так как костный мозг реагирует транзиторным ретикулоцитозом. Тромбозы являются показанием к пожизненной антикоагулянтной терапии варфарином. Излечить заболевание можно с помощью трансплантации костного мозга, но, учитывая связанный с этим вариантом лечения риск развития осложнений и летального исхода, данный метод остается в резерве для пациентов с тяжелой гипоплазией костного мозга или тяжелыми тромботическими осложнениями. В пилотном исследовании использование Экулизумаба – препарата антител к фактору C5 комплемента, нацеленного на подавление финальной стадии активации комплемента, – привело к сокращению эпизодов гемолиза и гемоглобинурии у пациентов с PNH. В настоящее время проводяться крупные рандомизированные контролируемые исследования этого нового препарата.
Оригинальный текст статьи:
Clinical Problem-Solving
More Than Meets the Eye
John S. Nguyen, M.D., Spyridon S. Marinopoulos, M.D., Bimal H. Ashar, M.D., and John A. Flynn, M.D.
N Engl J Med 2006; 355:1048-1052September 7, 2006
In this Journal feature, information about a real patient is presented in stages (boldface type) to an expert clinician, who responds to the information, sharing his or her reasoning with the reader (regular type). The authors' commentary follows.
A 61-year-old woman was hospitalized with a two-day history of palpitations and dyspnea. She was found to be in atrial fibrillation with a rapid ventricular response, and intravenous diltiazem and a heparin infusion were begun. Her condition improved, but on the third hospital day, she reported feeling weak and nauseated and began passing dark red urine. She did not have a urinary catheter, palpitations, dyspnea, back pain, abdominal pain, dysuria, or dizziness.
The presence of dark red urine usually suggests either gross hematuria or pigmenturia (hemoglobinuria or myoglobinuria). Although pigmenturia is commonly manifested by cola- or tea-colored urine, it may also cause urine that is dark red, maroon, or even bright cherry red. The finding of abundant red cells on microscopical examination would suggest hematuria, whereas the presence of hemoglobin on urinary dipstick testing without microscopic red cells would suggest pigmenturia. (An alternative way to distinguish these two conditions would be to centrifuge the urine: red urinary sediment indicates hematuria, and red supernatant indicates pigmenturia. In the latter case, the supernatant can be tested for hemoglobin with a dipstick.) The presence of red urine with a dipstick negative for hemoglobin and without red cells on microscopy would suggest a rare cause, such as beeturia or porphyria. If this patient does have gross hematuria, I would consider the possibility of a urinary tract infection, glomerular causes, cancer, or nephrolithiasis. If there is gross hematuria in the absence of a urinary tract infection, I would evaluate this patient's urinary tract with cystoscopy and computed tomography (CT) of the abdomen and pelvis.
The patient had a history of hypertension for which she took hydrochlorothiazide. She was otherwise in good health and took no other medications or herbal supplements. Her weight had not changed during the past several months. She had no history of foreign travel. She did not smoke, drink alcohol, or use illicit drugs. On examination, she was afebrile with a heart rate between 90 and 100 beats per minute, a blood pressure of 120/70 mm Hg, and respirations of 16 breaths per minute, with an oxygen saturation of 95% while breathing room air. Her lungs were clear. Cardiac examination was remarkable only for an irregular rhythm. Abdominal examination revealed no organomegaly, masses, or suprapubic tenderness. There was no costovertebral-angle tenderness. Rectal examination revealed no masses, and a guaiac test of stool was negative for occult blood. She had no skin lesions.
Hematuria resulting from a genitourinary cancer remains a possibility. The absence of fever, rigors, and costovertebral-angle tenderness makes pyelonephritis an unlikely diagnosis, although a lower urinary tract infection is still a possibility. Patients with rhabdomyolysis can present with pigmenturia (i.e., myoglobinuria). However, rhabdomyolysis is usually associated with myalgias, and the patient has not taken any medications associated with this complication. Another cause of dark red urine is hemoglobinuria as a result of brisk intravascular hemolysis. Glucose-6-phosphate dehydrogenase (G6PD) deficiency is possible, but there is no reported history of acute infection or exposure to a drug such as a sulfonamide or nitrofurantoin that is likely to precipitate an associated hemolysis. Other potential considerations would include march hemoglobinuria, valvular disease causing microangiopathic hemolysis, and paroxysmal nocturnal hemoglobinuria (PNH). There is no recent history of vigorous exercise or evidence of a heart murmur.
On the third hospital day, the white-cell count was 11,000 per cubic millimeter, as compared with 7000 per cubic millimeter at admission; the hematocrit was 30%, as compared with 35% at admission; the platelet count remained normal. The serum creatinine level was 1.8 mg per deciliter (160 μmol per liter), as compared with 0.9 mg per deciliter (80 μmol per liter) at admission. The ratio of the activated partial-thromboplastin time to a control value was 2.7 while the patient was receiving unfractionated heparin. The creatine kinase level was normal. Urinary dipstick testing revealed high levels of hemoglobin and protein (3+). Results of urine testing for myoglobin were negative. Microscopical examination of a urine specimen showed 2 to 3 red cells, 0 to 1 white cell, and 0 to 1 epithelial cell per high-power field, with few bacteria and no casts or crystals.
Despite the patient's grossly dark red urine and hemoglobin on dipstick testing, there were only 2 to 3 red cells per high-power field, suggesting acute hemoglobinuria or myoglobinuria as the cause. The finding of a normal creatine kinase level and negative results for myoglobinuria on urine testing leave hemoglobinuria as the only possibility. The presence of renal failure is worrisome, since hemoglobinuria may induce acute tubular necrosis through the formation of hemoglobin casts. The presence of proteinuria may reflect a transient process, such as infection or stress, or glomerular or tubular disease.
Bladder irrigation was initiated, and the patient's urine eventually became clear yellow. She remained afebrile. On the fourth hospital day, repeated laboratory testing revealed a serum creatinine level of 3.1 mg per deciliter (270 μmol per liter) and a blood urea nitrogen level of 29 mg per deciliter (10.4 mmol per liter). The hematocrit was 23%, the white-cell count was 7720 per cubic millimeter, and the platelet count was 203,000 per cubic millimeter. A peripheral-blood smear (Figure 1) showed normal-appearing white cells and no schistocytes, bite cells, cell fragments, or spherocytes.
Rapidly worsening anemia and renal failure are developing. In the absence of overt hemorrhage or aggressive fluid resuscitation, I am concerned about the possibility of massive hemolysis; the presence of hemoglobinuria indicates that the hemolysis is both intravascular and brisk. There is no report of a blood transfusion to suggest an acute hemolytic transfusion reaction. The patient's rising creatinine level may be due to pigment-induced acute tubular necrosis accompanied by extracellular volume depletion with renal ischemia. No mention has been made of possible exposure to a nephrotoxic agent or the intravenous administration of contrast material, but I would want to confirm that this information is correct. In the presence of worsening renal failure and ongoing hemolysis, hyperkalemia is a concern, and the potassium level should be monitored closely.
The potassium level was 4.7 mmol per liter, and the lactate dehydrogenase level 1610 IU per liter (normal, 122 to 220). Further evaluation revealed high levels of hemosiderin (4+) in the urine, normal serum levels of G6PD, a reticulocyte count of 4.3% (normal range, 0.5 to 1.8), undetectable haptoglobin levels, and negative direct and indirect Coombs' tests. The ferritin level was 30 μg per liter (normal range, 10 to 300), the serum iron level 38 μg per deciliter (7 μmol per liter; normal range, 50 to 170 μg per deciliter [9 to 30 μmol per liter]), the transferrin level 153 mg per deciliter (normal range, 200 to 400), and the iron saturation 20% (normal range, 20 to 55).
The markedly elevated lactate dehydrogenase level, undetectable haptoglobin levels, and hemosiderinuria confirm the diagnosis of intravascular hemolytic anemia. The normal G6PD levels and the absence of exposure to oxidative medications make G6PD deficiency unlikely, although normal levels may occasionally be seen in patients with this disease in the setting of acute hemolysis.
The patient has a ferritin level at the low end of the normal range, and iron deficiency, which can be a feature of chronic intravascular hemolysis, may be developing. Most hemolytic processes tend to occur extravascularly, with recovery of iron from the metabolized heme molecules through the reticuloendothelial system. However, massive intravascular hemolysis (as seen, for example, with march hemoglobinuria or PNH and in rare instances in patients with mechanical heart valves) may result in the liberation of a large amount of free hemoglobin intravascularly, which is then lost through renal excretion. The patient has been in the hospital and has no recent history of exertion, nor does she have a mechanical heart valve. Further inquiry into the patient's history is warranted to identify previous episodes of hemoglobinuria that would suggest the presence of PNH.
Renal ultrasonography showed no evidence of hydronephrosis, nephrolithiasis, or masses. The patient continued to receive fluids intravenously and a good urine output was maintained, but her serum creatinine level continued to increase and peaked at 3.5 mg per deciliter (310 μmol per liter). She had not received contrast material intravenously or nonsteroidal antiinflammatory drugs during or before her hospitalization.
The absence of hydronephrosis on ultrasonographic examination rules out urinary obstruction as a cause of this patient's illness. Intravenous hydration with normal saline should be continued to enhance renal perfusion and promote urinary output, as well as to prevent further renal damage from pigmenturia. Alkalinization of urine by the intravenous administration of sodium bicarbonate may provide renal protection by increasing the solubility of both myoglobin and hemoglobin and has been suggested in the management of nontraumatic rhabdomyolysis and pigmenturia. However, alkalinization carries a potential risk of hypocalcemia, and it is questionable whether it adds any benefit to volume replacement with saline alone.
Magnetic resonance angiography and venography revealed no evidence of renal-artery stenosis or renal-vein thrombosis. A renal biopsy was performed (Figure 2), and examination of the specimen revealed extensive acute tubular injury with hemoglobin casts. Iron deposition was noted in the tubular cells. On further questioning, the patient stated that she had had similar episodes of dark-colored urine intermittently over the past several years; these usually occurred in the setting of “a cold or the flu.”
The additional history of recurrent episodes of dark red urine and iron deposition in the tubular cells suggests a chronic relapsing course of hemolysis, which is characteristic of PNH. Examination of the renal-biopsy specimen indicates acute tubular necrosis from hemoglobin-cast deposition as a result of the patient's hemoglobinuria. Hemosiderin deposition of varying degrees within the proximal tubules has been found in patients with long-standing PNH. Mild chronic renal insufficiency and tubular concentrating defects may develop in patients with this disease. The diagnosis should be confirmed by tests showing the global deficiency of glycosylphosphatidylinositol (GPI) anchors or GPI-linked surface proteins such as CD55 or CD59.
Flow-cytometric analysis of the patient's peripheral blood revealed an abnormally low level of GPI-linked protein CD59 on red cells, monocytes, and granulocytes, which is diagnostic for PNH. During the remainder of her hospitalization, she had no further episodes of hemolysis; her serum creatinine level gradually increased to 3.0 mg per deciliter (270 μmol per liter), and she was subsequently discharged home in good health. At that time, her medications included a polysaccharide–iron complex twice daily. At a follow-up visit with her internist one month later, she had a serum creatinine level of 1.1 mg per deciliter (97 μmol per liter) and a hematocrit of 36.8%.
Commentary
Often, clinicians encounter new problems during the course of a patient's hospitalization that are unrelated to the initial reasons for admission. Hemoglobinuria and renal insufficiency developed in this patient while she was being treated with anticoagulants for atrial fibrillation. Because of the initial finding of a declining hematocrit and dark red urine, the first inclination might have been to attribute both to the anticoagulation therapy. Instead, further testing of the urine demonstrated hemoglobinuria rather than hematuria; the combination of this finding and renal failure prompted consideration of a pigment-induced nephropathy. This form of renal injury is seen more commonly with large crush injuries and rhabdomyolysis, in which myoglobin acts as a nephrotoxic agent.
Because it is larger than myoglobin and binds to haptoglobin, hemoglobin tends to be poorly filtered across the glomerulus, and much higher serum levels of hemoglobin than myoglobin are required to cause nephrotoxicity. Massive hemoglobinuria has occurred in cases of mismatched blood transfusions, and there have been scattered case reports of hemoglobinuria associated with various medications, including triamterene, rifampin, and methyldopa.1-3 Other causes of hemoglobinuria that may lead to renal failure include infections (typhoid fever, malaria, mumps, and clostridial sepsis), scorpion and snake bites, heavy exertion (march hemoglobinuria),4-6 and PNH. Had the history of this patient's hemoglobinuric episodes been obtained sooner, PNH could have been diagnosed earlier.
PNH is a rare disorder (with an estimated prevalence of 4 to 6 per million population) and is characterized by recurrent bouts of hemoglobinuria caused by intravascular hemolysis. Commonly, the urine is described as tea-colored or cola-colored, but it has been described in up to a third of patients as bright red.7 Typically, the urine is darkest in the morning and clears as the day progresses, hence, the origin of the term “nocturnal” in the name of this disorder. The paroxysmal nature of the disease originates from the intermittent complement-mediated destruction of red cells, which may be triggered by infections, surgery, blood transfusions, or vaccinations. In many cases of PNH, however, there are no discernible precipitating events. This patient's previous bouts of red urine were temporally associated with presumed viral infections, and thus may have been due to complement activation.
PNH is caused by a somatic mutation in the PIG-A gene located on the X chromosome. This gene encodes a protein necessary for the formation of GPI anchors on the surface of the various blood cell lines. Although several surface proteins are absent from the blood cells of patients with this disorder, the two most thoroughly studied are the complement-defense proteins CD55 and CD59, which normally inhibit membrane binding of C9. The absence of these surface proteins on red cells results in hemolysis when the complement cascade is activated. A relatively new technique for the diagnosis of PNH relies on a fluorescently labeled inactive variant of the protein aerolysin, which can selectively bind GPI anchors, to show the absence of GPI anchors on blood cells.8 Not all cells are affected; the larger the proportion of affected cells (known as the abnormal clone), the greater the severity of hemolysis and the risk of thrombotic complications.9
Intravascular hemolysis produces cell-free hemoglobin in the plasma, which then acts as a potent nitric oxide scavenger and can thus dramatically lower plasma levels of nitric oxide. The resulting decrease in plasma levels of nitric oxide may be directly responsible for most of the symptoms of PNH, including abdominal pain, esophageal spasms, erectile dysfunction, and venous thrombosis.10 Thrombosis commonly occurs in the abdominal venous system and can result in intestinal obstruction, ischemic colitis, gastrointestinal bleeding, or hepatic-vein thrombosis. Patients with PNH may have bone marrow hypoplasia with reduced numbers of erythroid and myeloid progenitor cells, and up to 13% may present with aplastic anemia.11
There are multiple case reports of PNH as a cause of acute renal failure; some cases have been severe enough to warrant temporary hemodialysis.12,13 Proteinuria of varying degrees, as seen in this case, has also been reported in patients with PNH and is presumed to be due to tubular disease. Renal failure ultimately occurs in approximately 5% of patients with PNH.14
Patients with PNH commonly have iron deficiency and may require oral iron supplementation. Occasionally, supplementation may initially result in a hemoglobinuric episode, as the bone marrow responds with brisk reticulocytosis. Thrombosis would be an indication for chronic anticoagulation with warfarin. Bone marrow transplantation may be curative, but given its associated risks of complications and death, it is reserved for patients with severe bone marrow hypoplasia or severe thrombotic complications. In a pilot investigation, the use of eculizumab, an antibody against C5 complement factor targeted to inhibit the final step of complement activation, resulted in reduced episodes of hemolysis and hemoglobinuria in patients with PNH.15 Larger randomized, controlled studies of this novel drug are currently under way.
No potential conflict of interest relevant to this article was reported.
Source Information
From the Department of Medicine, Johns Hopkins University School of Medicine, Baltimore.
Address reprint requests to Dr. Marinopoulos at 601 N. Caroline St., Suite 7143, Baltimore, MD 21287, or at smarino1@jhmi.edu.
 2015-04-20
2015-04-20 457
457