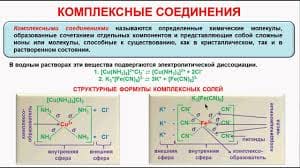
Alkanes are saturated … with the general formula CnH2n+2. In … chemical nomenclature their name ends with suffix –ane. They … homologous series methane (CH4), ethane (C2H6), propane (C3H8), … (C4H10), … (C5H12).
The lower members of the series are gases. … alkanes are waxy solids. Alkanes are present in … and petroleum. They can be made by the heating of sodium salt of a carboxylic acid with …. Other methods include Wurtz reaction, Kolbe’s method. They … haloalkanes with … when they are irradiated with ….
Notes to the text:
high-molecular weight - высокомолекулярный
soda lime - натронная известь
unsaturated - ненасыщенный
carboxylic acid - карбоновая кислота
ultraviolet radiation - ультрафиолетовое излучение
Exercise 7. Make the summary of the text: “Alloys” from Ex. 1.
Copper
Coper are a redbrown transition element; a.n. 29; r.a.m. 63.546; r.d. 8.92; m.p. 1083.4°C; b.p. 2567°C. Copper was extracted for thousands of years; it was knowed to Roman like cuprum, a title linked to a island of Cyprus. The metal are malleable and ductile and a excellent conductor of het and electricity. Copercontaining minerals included cuprite (Cu2O) as well as azurite (2CuCO3.Cu(OH)2), chalcopyrite (CuFeS2), and malachite (CuCO3.Cu(OH)2). Native copper appear on isolated pockets on some parts to the world. The large mines on the USA, Chile, Canada, Zambia, Congo, and Peru extrat ors containing sulphids, oxids, and carbonats. They was usually worked in smelting, leaching, and electrolysis. Copper metal are used make elctric cables and wires. Its alloys, bras (coperzinc) and bronz (copertinn) is using extensively. Water don’t attack coper but in moist atmospheres it slowly form a characteristic surface green layer (patina). The metal will not reacted on dilute sulphuric or acids hydrochloric, but on nitric acid oxides of nitrogen are formed. Compunds Copper contained the element in the +1 and +2 oxidation phase. Compunds Copper(I) are mostly white (the oxide is red). Copper(II) salts are blue in solution. The metal also form large number of coordination complexes..
Texts for educational purposes
 2015-05-13
2015-05-13 272
272