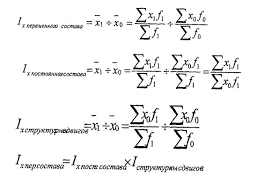
The soft blue-white glow of fluorescent lamps can be seen everywhere from the tops of multistoried buildings to the depths of the underground system. Many people know that fluorescent lamps are among our most efficient sources of light, and that they operate with a mercury vapour arc. But few realize that over 80 per cent of the radiation produced by that arc is in the ultraviolet region and invisible to the human eye, and that every effort is made to keep as much energy as possible in the invisible ultraviolet end of the spectrum.
The visible light actually comes from chemical compounds coated on the inside of the glass tube. These compounds called phosphors have the property of emitting visible light when they are excited by ultraviolet radiation. They have been termed "light transformers" because, of their ability to absorb energy at- one wavelength and radiate it at another.
The production of the necessary ultraviolet arc in the fluorescent lamp depends upon ionization. Here is how it is done. The free electrons in the gas are accelerated by an applied voltage, and each time a collision occurs between an electron and a gas molecule, one or more additional electrons are displaced. These electrons, in turn, are accelerated enough to repeat the process on other molecules, and a chain reaction takes place.
As each molecule returns to a stable state, it gives off its excess energy in the form of radiation. It is the frequency of the radiation that determines whether visible or invisible light will be obtained. The pressure of the gas sealed in the lamp is adjusted very carefully so that nearly all of the radiation occurs at one given ultraviolet wavelength most suitable for excitation of the tube's phosphor coating.
Each chemical compound in the phosphor coating radiates light at a certain wavelength. For instance, zink silicate releases its radiation as green light, cadmium borate radiates a pink colour, and calcium tungstate (a salt of tungstic acid) when excited gives off blue light. By carefully blending these and other components, almost any desired colour can be obtained.
 2015-06-28
2015-06-28 513
513