The relationship between the rate of a chemical reaction and the concentration of the reactants is shown by the rate equation of the reaction.
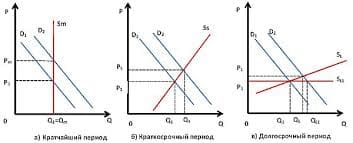
Consider the reaction A + 3B à 2C + 4D
The rate of this chemical reaction is given by the equation
Rate = k[A]x[B]y
[A] is the concentration of A, and [B] is the concentration of B.
x and y are the orders of reaction with respect to A and B respectively.
| The order of reactionwith respect to a given reactant is the power of that reactant's concentration in the rate equation. |
The sum of these powers, in this case x + y, is known as the overall order of reaction:
| The overall order of reaction is the sum of the powers of the reactant concentrations in the rate equation |
k is the rate constant of the reaction.
| The rate constant is the constant of proportionality in the rate equation. |
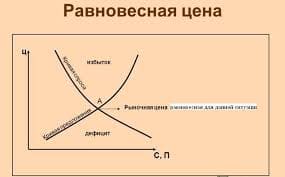
1. Rate of reaction
During a chemical reaction, the concentration of the reactants decreases and the concentration of the products increases.
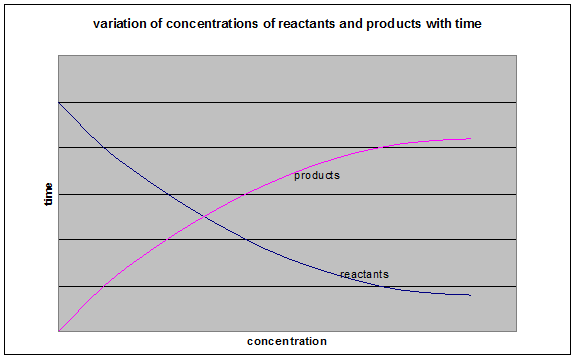
| The rate of a reaction is the decrease in concentration of reactants per unit time, or the increase in concentration of products per unit time. |
The units of rate of reaction are moldm-3s-1.
 2015-07-14
2015-07-14 233
233