If the concentrations in the different experiments are not simple whole number ratios of each other, it is not easy to compare the concentrations and rates. The order of reaction with respect to each reactant can be deduced by plotting a graph of concentration vs initial rate.
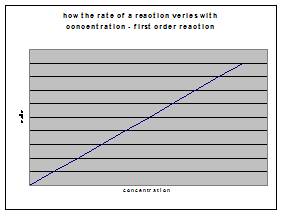
a) first-order reactions
If Rate = k[A], then a plot of initial rate against initial concentration will be a staight line through the origin of gradient k:
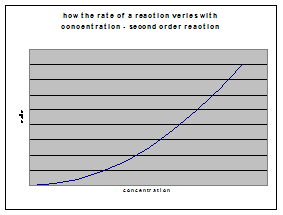
b) second-order reactions
If rate = k[A] 2, then a plot of initial rate against initial concentration will be a curve through the origin.
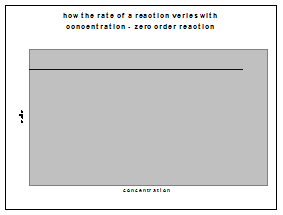
c) zero order reactions
If rate = k, then a plot of initial rate against initial concentration will be a horizontal line:
An even better method is to plot log (rate of reaction) against log (concentration). This should always give a straight line, the gradient of which is the order of reaction.
 2015-07-14
2015-07-14 263
263