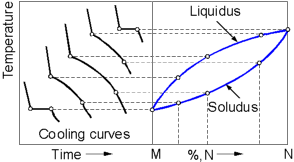
 A binary phase diagram is composed from the cooling curves of alloys with various compositions. For an alloy with a particular composition there are two points on the curve where a cooling rate is affected. The first point corresponds to the temperature at which the alloy begins to solidify. On the phase diagram this point belongs to the Liquidus line. The second point corresponds to the temperature at which the entire liquid has solidified. On the phase diagram this point belongs to the Solidus line.
A binary phase diagram is composed from the cooling curves of alloys with various compositions. For an alloy with a particular composition there are two points on the curve where a cooling rate is affected. The first point corresponds to the temperature at which the alloy begins to solidify. On the phase diagram this point belongs to the Liquidus line. The second point corresponds to the temperature at which the entire liquid has solidified. On the phase diagram this point belongs to the Solidus line.
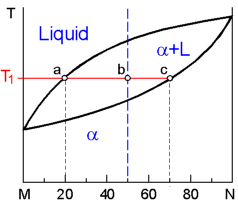 This diagram dispays the complete liquid and solid solubility of two component otherwise known as a binary isomorphous system. This occurs when the components have the same crystal structure and approximately the same radii, electro negativity and valence. For instance, systems of Ni-Cu and Ag-Au exhibit this type of diagram.
This diagram dispays the complete liquid and solid solubility of two component otherwise known as a binary isomorphous system. This occurs when the components have the same crystal structure and approximately the same radii, electro negativity and valence. For instance, systems of Ni-Cu and Ag-Au exhibit this type of diagram.
At the temperature T1 for an alloy of 50%M + 50%N:
Composition of solid solution a is determined at point c - 30%M +70%N.
Composition of Liquid is determined at point a - 80%M +20%N.
% Liquid = bc/ac • 100% = (70-50)/(70-20) · 100 % = 40%
% a = ab/ac • 100% = (50-20)/(70-20) · 100 % = 60%
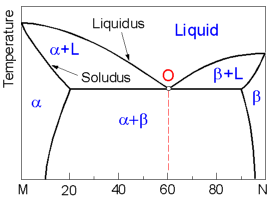 The following diagram displays the complete liquid and limited solid solubility of two components otherwise known as the binary eutectic system. There are two solid phases (solid solutions): a - rich in the component M and b - rich in the component N.
The following diagram displays the complete liquid and limited solid solubility of two components otherwise known as the binary eutectic system. There are two solid phases (solid solutions): a - rich in the component M and b - rich in the component N.
At point O (Eutectic point) three phases (one liquid and two solid phases) coexist simultaneously at the eutectic composition and temperature.
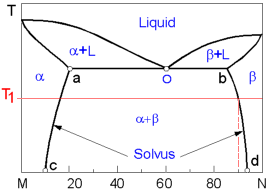 Line bd or the Solvus line protrays the change of the maximum concentration of component M in component N. At the temperature T1 the maximum concentration M in N is 10%. The highest possible solubility of the component M in component N (and vise verse) is found at the eutectic temperature. On the diagram this constant temperature line goes through the eutectic point O.
Line bd or the Solvus line protrays the change of the maximum concentration of component M in component N. At the temperature T1 the maximum concentration M in N is 10%. The highest possible solubility of the component M in component N (and vise verse) is found at the eutectic temperature. On the diagram this constant temperature line goes through the eutectic point O.
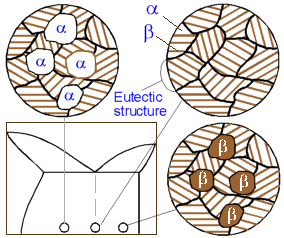 All alloys with composition along line ab comprise the eutectic structure which alternates layers of a and b phases. The closer the alloy to the eutectic composition, the higher amount of the eutectic it contains. The solidified alloys within line ab is a mixture of a grains precipitated prior to the eutectic reaction and grains of the eutectic. While the alloys on the right from point O - mixture of b and the eutectic grains.
All alloys with composition along line ab comprise the eutectic structure which alternates layers of a and b phases. The closer the alloy to the eutectic composition, the higher amount of the eutectic it contains. The solidified alloys within line ab is a mixture of a grains precipitated prior to the eutectic reaction and grains of the eutectic. While the alloys on the right from point O - mixture of b and the eutectic grains.
 2015-08-13
2015-08-13 469
469