The electrochemical series ranks the general resistance of metals to corrosion. The more negative the standard Emf (Electromotive force) potential, the more easily the material will oxidize.
The monocrystal structure shows a higher resistance to corrosion than the same metal with polycrystalline structure. The smaller the size of grains, the more the material is prone to corrosion damages.
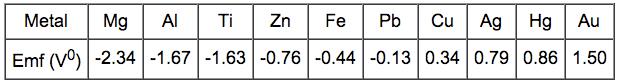
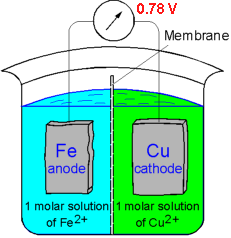 If two metals are electrically connected and immersed in a solution of their own ions the EMF potential determines which material will corrode. Iron dissolves in the electrolyte because iron has electrode potential (-0.44 V) lower than that of copper (0.33 V). Consquently, the copper deposits on the cathode. The magnitude of the voltage driving the dissolution of iron is found to be:
If two metals are electrically connected and immersed in a solution of their own ions the EMF potential determines which material will corrode. Iron dissolves in the electrolyte because iron has electrode potential (-0.44 V) lower than that of copper (0.33 V). Consquently, the copper deposits on the cathode. The magnitude of the voltage driving the dissolution of iron is found to be:
DV = V1 - V2 = 0.34 - (-0.44) = 0.78 V
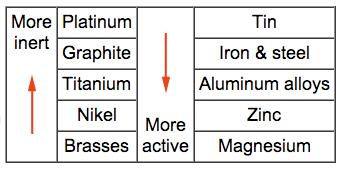 Galvanic corrosion occurs when dissimilar metals are placed in assembly within a corrosive electrolyte (e.g. sea water). This results in one of the metals becoming anodic and corroding at faster rate than normal. The other metal is the cathode responds with a decrease in corrosion rate. The Galvanic series is useful for selecting materials to be joined. Materials towards the bottom of the table are more active (anodic) and will corrode at a faster rate than those above them. In addition, the closer two metals are in the table the weaker the corroding effect.
Galvanic corrosion occurs when dissimilar metals are placed in assembly within a corrosive electrolyte (e.g. sea water). This results in one of the metals becoming anodic and corroding at faster rate than normal. The other metal is the cathode responds with a decrease in corrosion rate. The Galvanic series is useful for selecting materials to be joined. Materials towards the bottom of the table are more active (anodic) and will corrode at a faster rate than those above them. In addition, the closer two metals are in the table the weaker the corroding effect.
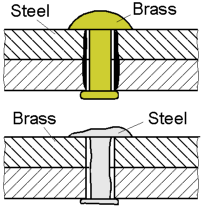 Corrosion rate depends on the relative areas of the anode and cathode. When the surface area of the anodic metal is smaller than that of the cathode the resulting corrosion is rapid. Consequently, the corrosion rate is slow when a larger anode is connected to a small cathode.
Corrosion rate depends on the relative areas of the anode and cathode. When the surface area of the anodic metal is smaller than that of the cathode the resulting corrosion is rapid. Consequently, the corrosion rate is slow when a larger anode is connected to a small cathode.
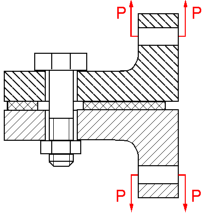 The bolt displayed under a constant load will corrode at a greater rate than one that is unloaded. This is due to regions of a high local stress being anodic to those of a lower stress. The combined action of a sufficient applied tensile stress and an aggressive environment can cause the cracking of a part.
The bolt displayed under a constant load will corrode at a greater rate than one that is unloaded. This is due to regions of a high local stress being anodic to those of a lower stress. The combined action of a sufficient applied tensile stress and an aggressive environment can cause the cracking of a part.
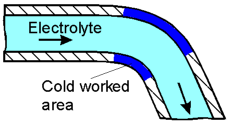 Areas of metals subjected to cold working are rich in dislocations and therefore constantly under stress. This results in them being anodic to the less stressed regions and accelerates corrosion.
Areas of metals subjected to cold working are rich in dislocations and therefore constantly under stress. This results in them being anodic to the less stressed regions and accelerates corrosion.
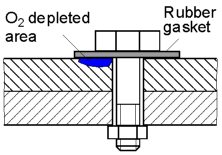 The flow of oxygen to the area under the gasket is restricted and therefore its concentration is low. This area will be anodic and corrode faster than the oxygen rich areas.
The flow of oxygen to the area under the gasket is restricted and therefore its concentration is low. This area will be anodic and corrode faster than the oxygen rich areas.
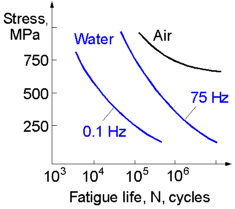 For a given fatigue life the influence of a corrosive environment on the fatigue strength of metals increases if the frequency decreases. This means that a structure loaded at a lower frequency will sustain less cycles to fracture at a given applied stress. The picture shows S-N curves of carbon steel tested in several mediums. All metals and alloys cyclically loaded under a corrosive environment do not exhibit a endurance (fatigue) limit. Which means that a structure exploited under such conditions will finally break even if applied stress is very low.
For a given fatigue life the influence of a corrosive environment on the fatigue strength of metals increases if the frequency decreases. This means that a structure loaded at a lower frequency will sustain less cycles to fracture at a given applied stress. The picture shows S-N curves of carbon steel tested in several mediums. All metals and alloys cyclically loaded under a corrosive environment do not exhibit a endurance (fatigue) limit. Which means that a structure exploited under such conditions will finally break even if applied stress is very low.
 2015-08-13
2015-08-13 801
801