Scientists have studied the nature of electricity for hundreds of years. They have determined that it is a form of energy. Energy can do work. Tiny, invisible, electrically charged particles are present in atoms, the basic building blocks of all matter. The amount and kind of electric particles determine the properties of an atom.
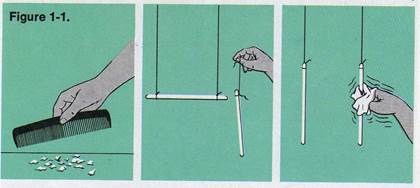 |
ACTIVITY. Run a comb through dry hair
several times. Then try to pick up bits
of paper with the comb. What happens?
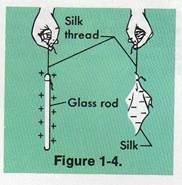
Rub a glass rod with a piece of silk.
Hang the rod horizontally with silk threads. Rub
a second glass rod in the same way. Hang it by a
silk thread and bring it near the first rod.
What happens?
Using silk threads, hang two plastic rods separately.
Rub the two rods with wool. Bring them close
together. Do they repel or attract each other?
Now bring a suspended plastic rod rubbed with
wool near a suspended glass rod rubbed with silk.
Does glass repel glass? Does plastic normally repel plastic? Does glass normally attract plastic? Explain what you observe.

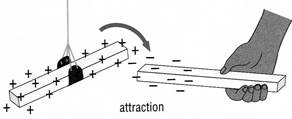
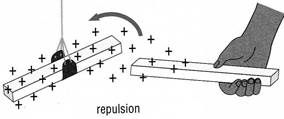
Some materials when rubbed gain an
electric charge. The charge can be positive (+) or
negative (-). When two rods have the same charge,
the rods repel each other. When two rods have different
charges, the rods attract each other. For this reason,
charged glass and plastic rods attract each other.
The charge on the glass rod is opposite to the charge
on the plastic rod. Like charges repel each other.
Opposite charges attract each other.
An atom contains equal numbers of protons and
electrons. For example, an oxygen atom has 8 protons
and 8 electrons. A proton is a positively charged
particle in the nucleus of an atom. An electron is a
negatively charged particle outside the nucleus. Since
the number of negative charges in an atom is equal
to the number of positive charges, an atom is neutral.
If a body gains electrons, the result will be more
electrons than protons. The object is then
 negatively charged. If an object loses electrons, the result will be more protons than electrons. Then the object is positively charged.
negatively charged. If an object loses electrons, the result will be more protons than electrons. Then the object is positively charged.
ACTIVITY.
Attach a silk thread to a glass rod. Attach a silk thread to a piece of silk cloth.
Rub the rod with the silk. Suspend the rod and silk cloth separately. Bring the silk cloth near
the rod. What do you observe?
If you rub a glass rod with silk, the glass rod loses electrons to the silk. The rod
becomes positively charged. The silk becomes negatively charged. Opposite charges attract,
so the rod and silk attract each other.
When you rub a plastic rod with wool, the plastic rod gains electrons from the wool.
Thus, the plastic rod becomes negatively charged. The wool becomes positively charged.
Will the wool and the plastic rod attract each other? Why?
Charged bodies have static electricity, or electricity at rest. There are two kinds of static electricity. Positive static electricity results when an object loses electrons. It has a positive charge. Negative static electricity results when an object gains electrons. It has a negative charge.
Scientists often use models to explain what they see. In science, a model isn't just a small copy of some larger thing. Instead, a model is a way of explaining observations. The model used in explaining charging with electricity is partly described by the following list:
1) There are two different kinds of electric charges positive and negative.
2) When an object has more positive than negative charges, it is positively charged. If it has more,negative than positive charges, it is negatively charged. If it has equal numbers of positive and negative charges, it is neutral, or not charged.
3) Rubbing can cause negative charges to move from one solid object to another solid object. Positive charges do not move from one solid object to another.
4) Two negatively charged objects or two positively charged objects repel each other. But a negatively charged object and a positively charged object attract each other. Sometimes, this is stated as "like charges repel and unlike charges attract."
5)  Materials in which negative charges move about easily are called conductors. Metals are good conductors. Materials in which negative charges do not move about easily are poor conductors, or insulators. Rubber is an insulator.
Materials in which negative charges move about easily are called conductors. Metals are good conductors. Materials in which negative charges do not move about easily are poor conductors, or insulators. Rubber is an insulator.
You may see examples of static electricity when
(a) charged clouds produce lightning
(b) someone wearing rubber-soled shoes walks over a wool rug
(c) a driver in a wool coat rubs against plastic car seats
(d) a nylon dress comes out of a clothes dryer
(e) a blown-up balloon is rubbed against a wool sweater.
PROBLEMS
1)Why may static electricity be produced in each example
given above?
2)  Name three other examples of static electricity.
Name three other examples of static electricity.
3)When you comb your hair, the comb may gain a negative charge.
4)Where do the added electrons come from?
 | |||
 |
charge – заряд like charges – однойменні заряди
opposite charges – різнойменні заряди attract – притягувати
repel – відштовхувати nucleus - ядро
rub – тертя conduction – провідність
conductor – провідник insulator - ізолятор
 2015-10-14
2015-10-14 546
546