So:
The absorbance of a solution is proportional to concentration of light-absorbing substance and a thickness of a layer
Or
The relationship between a sample’s absorbance and the concentration of the absorbing species

where: A – optical density (absorbance), ε – the molar absorptivity, C – concentration (molarity)
Additivity of optical densities
Beer’s law can be extended to samples containing several absorbing components provided that there are no interactions between the components. Individual absorbances, Ai, are additive. For a two-component mixture of X and Y, the total absorbance, A tot, is


So
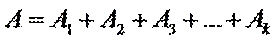

A = l(e1С1 + e2С2 + …ekСk)
| permeate | e (the molar absorptivity) |
| Iron (ІІІ) rhodanate | 103 |
| Complex Ti with H2O2 | 103 |
| Complex Ti with chromotrope acid | 105 |
| Complex Cu with ammonia | 5 ×102 |
| Complex Cu with dithizon | 5 ×104 |
| Complex Al with aluminon | 1,7 ×104 |
| Complex Al with 2-stilbazole | 3,5 ×104 |
 2017-11-01
2017-11-01 275
275