Text
Electric cell is a device that stores electric energy in the form of
chemical energy. All cells consist of an electrolyte (solution containing ions), positive electrode and negative electrode. Electricity is generated when the negative electrode (cathode) reacts with the electrolyte. During the reaction electrons are released. They move to the positive electrode (anode). The electric energy is released when a conductor is connected be- tween the terminals of the cell. When the cell has no power to release elec- tricity (when electrolyte has reacted fully), the cell is said to be discharged. Some types of cells can be recharged by passing current in reverse direc- tion. This type of rechargeable cells is also called storage cell or secondary cell. Primary cells cannot be recharged.
Let’s consider some basic types of electric cells.
Alkaline dry cells
|
torches. They can supply quite large currents for long periods.
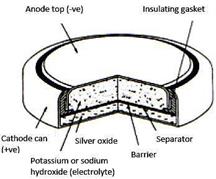
Mercury cells
|
used in wristwatches, calculators, etc. In these cells the negative electrode consists of zinc, while the positive electrode is made of mercuric oxide
|
 2021-11-09
2021-11-09 298
298