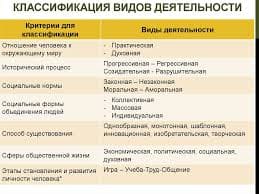
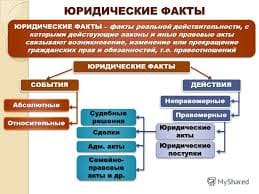
Прочитайте и переведите текст
Molecules are chemical units composed of one or more atoms. The simplest molecules contain one atom each; for example, helium atoms (one atom per molecule) are identical with helium molecules. Oxygen molecules (O2) are composed of two atoms, and ozone (O3) of three. Molecules may contain several different sorts of atoms. Water (H2O) contains two different kinds, hydrogen and oxygen, and dimethyl amine ((CH3)2NH) has three kinds. Molecules of many common gases (hydrogen H2, oxygen O2, nitrogen N2, and chlorine C12) consist of two atoms each.
Not all molecules are molecular in structure. Some are atomic and many are ionic. Molecular substances are characterized by low boiling points and poor conductivity of electricity when dissolved or melted. Gases are generally molecular, and so are many liquids, and some solids. All compounds of hydrogen and non-metallic oxides are molecular. These compounds are considered to be bonded by a force called a covalent bond (or bonds) which consists of one or more shared electron pairs.
The size of molecules, especially of the smaller ones, is so tiny that to make a meaningful comparison is rather difficult. Let us assume that the water molecules in a cup of water are dyed so that they can be identified. If this cup had been thrown into the ocean 2,000 years ago, these molecules would have become distributed evenly in all bodies of water on the earth.
A cup of water taken at random from nearest supply, would give you at least one hundred of the original dyed molecules. The exceedingly large number of molecules of water in a cup is, of course, directly related to the fact that each molecule is exceedingly small. Molecules are too small to be visible in ordinary light. The most powerful and best illuminated optical microscope has failed to reveal molecules by direct vision, although it is claimed that they have been resolved in the electron microscope.
Перескажите текст. Выскажите свое мнение по следующим темам:
- Kinds of Molecules.
- Molecular Composition and Structure.
- Definition of a Molecule.
Test 8
1. Bohr’s atomic theory … since 1913.
a) has known
b) had been known
c) know
d) has been known
2. An electron microscope is a very useful … for molecular investigations.
a) device
b) equipment
c) tool
d) instrument
3. To compare the size of molecules is rather …
a) easy
b) difficult
c) different
d) light
4. A molecule of water is a certain … of hydrogen and oxygen atoms.
a) relation
b) definition
c) combination
d) motion
5. Molecules may be molecular, atomic or ionic in ….
a) combination
b) size
c) content
d) Structure
6. Molecules are too small and cannot … by ordinary means.
a) be seen
b) seen
c) be see
d) see
7. Molecules are too small to be visible in … light.
a) day
b) sun
c) ordinary
d) usual
8. Molecules are … as the smallest particles.
a) considered
b) regarded
c) seen
d) known
9. A molecule of a compound … at least two different atoms.
a) contains
b) consists
c) makes
d) has
10. We call the atomic hypothesis a ….
a) theory
b) science
c) method
d) system
Unit 9
 2015-02-27
2015-02-27 724
724