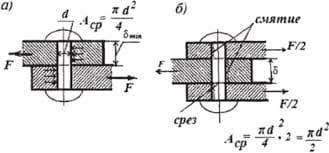
The most intense fluorescence is found in compounds containing aromatic group with low-energy π→π∗ transitions. A few aliphatic, alicyclic carbonyl, and highly conjugated double-bond structures also exhibit fluorescence as well. Most unsubstituted aromatic hydrocarbons fluoresce in solution too. The quantum efficiency increases as the number of rings and the degree of condensation increases. Simple heterocycles such as the structures listed below do not exhibit fluorescence.
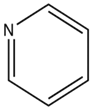

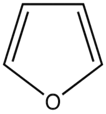
 Pyridine Pyrrole Furan Thiophene
Pyridine Pyrrole Furan Thiophene
With nitrogen heterocyclics, the lowest energy transitions is involved in n→π∗ system that rapidly converts to the triplet state and prevents fluorescence. Although simple heterocyclics do not fluoresce, fused-ring structures do. For instance, a fusion of a benzene ring to a hetercyclic structure results in an increase in molar absorptivity of the absorption band. The lifetime of the excited state in fused structure and fluorescence is observed. Examples of fluorescent compounds is shown below.
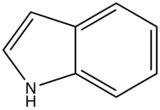
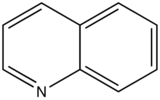
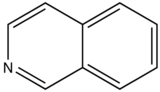
quinoline
Benzene ring substitution causes a shift in the absorption maxima of the wavelength and changes in fluorescence emission. The table below is used to demonstrate and visually show that as benzene is substituted with increasing methyl addition, the relative intensity of fluorescence increases.
| Compound | Structure | Wavelength of Fluorescence (nm) | Relative intensity of Fluorescence |
| Benzene | 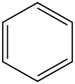
| 270-310 | |
| Toluene | 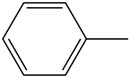
| 270-320 | |
| Propyl Benzene | 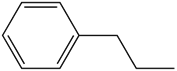
| 270-320 |
Table 2. Relative intensity of fluorescence comparison with alkane substituted benzenes.
The relative intensity of fluorescence increases as oxygenated species increases in substitution. The values for such increase is demonstrated in the table below.
| Compound | Structure | Wavelength of Fluorescence (nm) | Relative intensity of Fluorescence |
| Phenol | 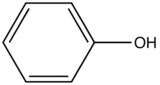
| 285-365 | |
| Phenolate ion | 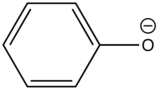
| 310-400 | |
| Anisole | 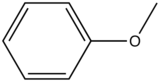
| 285-345 |
Table 3. Relative intensity of fluorescence comparison with benzene with oxygenated substituted benzene
Influence of a halogen substitution decreases fluorescence as the molar mass of the halogen increases. This is an example of the “heavy atom effect” which suggest that the probability of intersystem crossing increases as the size of the molecule increases. As demonstrated in the table below, as the molar mass of the substituted compound increases, the relative intensity of the fluorescence decreases.
| Compound | Structure | Wavelength of Fluorescence (nm) | Relative intensity of Fluorescence |
| Fluorobenzene | 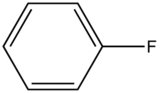
| 270-320 | |
| Chlorobenzene | 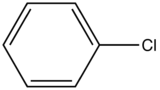
| 275-345 | |
| Bromobenzene | 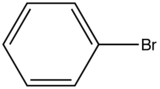
| 290-380 |
Table 4. Relative intensity fluorescence comparison with halogen substituted compounds
In heavy atom substitution such as nitro derivatives or heavy halogen substitution such as iodobenzene, the compounds are subject to predissociation. These compounds have bonds that easily rupture that can then absorb excitation energy and go through internal conversion. Therefore, the relative intensity of fluorescence and fluorescent wavelength is not observed and this is demonstrated in the table below.
| Compound | Structure | Wavelength of Fluorescence (nm) | Relative intensity of Fluorescence |
| Iodobenzene | 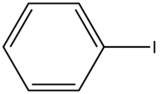
| None | |
| Anilinium ion | 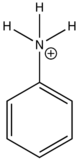
| None | |
| Nitrobenzene | 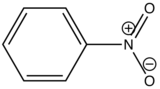
| None |
Table 5. Relative fluorescent intensities of iodobenzene and nitro derivative compounds
Carboxylic acid or carbonyl group on aromatic ring generally inhibits fluorescence since the energy of the 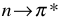 transition is less than
transition is less than 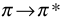 transition. Therefore, the fluorescence yield from
transition. Therefore, the fluorescence yield from 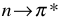 transition is low.
transition is low.
| Compound | Structure | Wavelength of Fluorescence (nm) | Relative intensity of Fluorescence |
| Benzoic Acid | 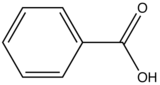
| 310-390 |
Table 6. Relative fluorescent intensity of benzoic acid
 2015-08-21
2015-08-21 460
460