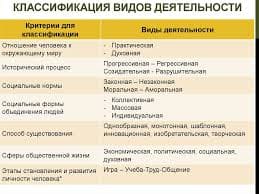
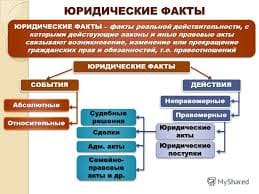
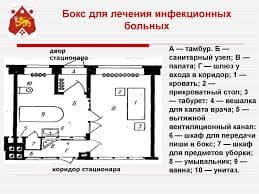
Give an oral summary of the text in your own words omitting all unnecessary details.
Give your own conclusion to the text.
Translate the following text into English.
Каучук (caotchouc) існує так довго, як і сама природа. Знайдені закам’янілі залишки каучуконосних дерев мають вік близько трьох мільйонів років.
Перше знайомство європейців із натуральним каучуком відбулося п’ять століть тому, а в США речі з каучуку набули популярності в 1830-х роках, гумові (rubber) пляшки й взуття, виготовлені південноамериканськими індіанцями, продавалися у великих кількостях.
1839 року американський винахідник Чарльз Гудьєр (Charles Goodyear) дослідив, що нагрівання каучуку з сіркою усуває його неприємні властивості. Він поклав на піч шматок накритої каучуком тканини, на яку було нанесено шар сірки. За деякий час він побачив шкірястий матеріал – гуму. Цей процес було названо вулканізацією.
Відкриття гуми привело до широкого її застосування: до 1919 року випустили на ринок більше 40 000 різноманітних виробів із гуми.
TEXT 18
STRATOSPHERIC OZONE
Learn the new vocabulary:
| chlorine | хлор |
| to be spilled | виливатися, розливатися |
| to emit | випускати, випромінювати |
| to prevent | попереджати, запобігати |
| to contrive | вигадувати, винаходити |
| eventually | зрештою |
| to absorb ultraviolet sunlight | поглинати ультрафіолетове проміння |
| disturbing change | хвилюючі зміни |
| to occur | ставатися |
| emission | виділення, поширення |
| to penetrate | проникати |
| decade | десятиліття |
1. Read and translate the text:
Ordinarily there is very little chlorine in the stratosphere. Chlorine gas is sometimes spilled in industrial or shipping accidents, but this gas reacts strongly with almost any water-drop or particle it touches and, as a result, is used up long before it can diffuse upward. Ocean waves throw up small droplets of salty water, some of which evaporate, leaving salt particles in the air. Although these particles contain chlorine, the chance that one of them will get as high in the atmosphere as the ozone layer is small, since salt is very soluble and these particles are readily washed out of the air by the rain. Some biological systems emit methyl chloride, a gas that contains chlorine. But this gas reacts fairly rapidly with other substances, and most of it disappears before it can diffuse to the stratosphere. Thus, strong barriers prevent chlorine from reaching high in the atmosphere, unless people contrive to put it there.
Damage to the layer of ozone in the high atmosphere by human activity is complex, esoteric, and completely invisible to anyone but the scientists who are studying the issue. Yet, around the world, people who twenty years ago had never heard the word ozone are now worried about its disappearance.
Two of these substances, CFC-11 and CFC-12, have proved so valuable in a number of applications that more than 20 million tons have been manufactured worldwide. Most of these 20 million tons still exist and either escaped to the atmosphere or eventually will. Once in the air, these substances mix and diffuse, finally reaching all parts of the atmosphere. Those CFC molecules that find themselves in the stratosphere are subjected to intense ultraviolet radiation from the Sun; they split apart into smaller fragments, releasing chlorine. The chlorine then starts a new career as a catalyst in the reactions that destroy ozone.
Ozone plays an important role in the high atmosphere in addition to screening out UV-B. By absorbing ultraviolet sunlight, ozone deposits the heat associated with this light into that level of the atmosphere, thus creating a layer much warmer than those immediately below. The stable region so created is the stratosphere. It is in this stable layer that disturbing changes are occurring. As scientists' understanding of the chemical reactions that create and destroy ozone increased, it became clear that relatively small quantities of some substances could change these reactions and hence the amount of ozone in the stratosphere, provided those substances were placed in the high atmosphere. And chlorine, an effective chemical catalyst that can change ozone into normal oxygen, is appearing in rapidly increasing concentrations in the atmosphere.
If we did wish, for some reason, for chlorine at the Earth's surface to move into the atmosphere, we would have to arrange for the emission at the surface of the Earth of a chlorine-containing gas. We would, in addition, have to find a chlorine-containing gas that did not react readily with anything, one that was not very soluble, and one that, upon reaching the stratosphere, could be broken down to release free chlorine only by the action of strong ultraviolet light. (If it were broken down too soon, by sunlight that penetrates low into the atmosphere, the free chlorine would react with something and be removed). The described properties would also make the gas extremely useful here at the surface of the Earth, and people have worked hard to create such a substance.
Laboratory scientists created such substances decades ago. They are called chlorofluorocarbons, indicating that they contain carbon, fluorine, chlorine, and sometimes hydrogen. The name is frequently abbreviated to CFC, and a numbering scheme is used to tell how much of each element is in the molecule of the particular CFC under discussion. CFC-12, for example, has one atom of carbon, no atoms of hydrogen, two atoms of fluorine (and, by implication, two atoms of chlorine) in each molecule.
Entitle each paragraph so as to make a plan and write down the sentence(s) that express the main idea(s) of each paragraph.
 2018-02-13
2018-02-13 698
698