The dependence of the viscosity of oil products on temperature is a very important characteristic both in oil refining technology (pumping, heat transfer, sludge, etc.) and in the use of oil products (drain, pumping, filtering, lubricating rubbing surfaces, etc.).
With decreasing temperature, their viscosity increases. The figure shows the curves of changes in viscosity depending on temperature for various lubricating oils (Fig. 8.7.5).
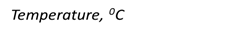


Fig. 8.7.5. Dependence of viscosity on temperature:
1 – strong dependence; 5 – less strong
Common to all oil samples is the presence of temperature ranges in which a sharp increase in viscosity occurs.
 There are many different formulas for calculating viscosity as a function of temperature, but Walter's empirical formula is the most commonly used:
There are many different formulas for calculating viscosity as a function of temperature, but Walter's empirical formula is the most commonly used:

Double logarithm of this expression, we get:
lg (lg (100⸱ υt + 0.8)) = A – B ⸱ lgT, (8.7.6)
where:
υt – kinematic viscosity, m2/c;
T – temperature, K;
A – lg (lg K);
B – constant value for a given substance.
A and B constant values for a given substance are determining the steepness of the curve of the dependence of viscosity on temperature. In logarithmic coordinates, they characterize the slope of the line.
According to this equation, a nomogram was compiled (Appendix 5). The nomogram is suitable for determining the viscosity of all types of liquid petroleum products.
For petroleum lubricating oils, it is very important during operation that the viscosity is as little as possible dependent on temperature, as this ensures good lubricating properties of the oil over a wide temperature range. This means, according to the Walther’s formula, that the lower the coefficient B, the higher the quality of the oil. This property of oils is called viscosity index, which is a function of the chemical composition of the oil.
For different hydrocarbons, the viscosity varies depending on the temperature to different degrees. Aromatic hydrocarbons have the highest dependence (high value B), and the lowest – alkanes. Naphthenic hydrocarbons are close to alkanes.
Since the viscosity of the temperature varies according to the hyperbolic law, it is more convenient to go to the logarithmic coordinates (Fig. 7.8.6). Then the dependence will be direct. The viscosity index of alkanes is taken for 100, aromatics – for 0. The interval between the viscosity values at given temperature is divided into 100 parts.
Any mixture of hydrocarbons falls into the zone limited by these reference molecules. According to the number of divisions cut off by the studied oil product (X parts), the viscosity index of the test mixture is assigned (VI).
There are various mathematical methods for determining the viscosity index (VI).
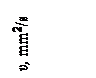


a b
Fig. 8.7.6. Calculation of dependence of viscosity on temperature:
a – inCartesian coordinates; b – in logarithmic coordinates
VI is determined by two values of kinematic viscosity at 40 and 100°C. VI is calculated by the formula:
|

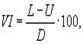
where:
L – kinematic viscosity of a petroleum product with VI = 0 at 40°C having the same kinematic viscosity at 100°C as the tested petroleum product at 100°C, mm2/s;
U – kinematic viscosity of the tested petroleum product at 40 °C;
H – kinematic viscosity a petroleum product with VI = 100 at 40 °C of having the same kinematic viscosity at 100 °C as the tested petroleum product 100°C, mm2/s;
Y – kinematic viscosity at 100 °C of the oil product whose viscosity index is to be determined (D=L – H), mm2 / s.
For all oils whose viscosity at 100 degrees is less than or equal to 70 mm2/s (υ100 < 70), the values L and D are determined from the table (Appendix 6).
If the oil has a viscosity at 100 degrees greater than 70 mm2/s (υ 100 > 70) mm2/s, the values of the parameters included in the Formula 7.8.7 determined by the equations:
L = 0.8353 Y2 + 14.67 Y – 216
D = 0.6669 Y2 + 2.82 Y – 119
If the oil has a viscosity at 100 degrees less than 2 mm2/s (υ100 < 2 mm2/s):
L = Y (1.5215 + 0.7092 Y)
D = Y (0.17129 + 0.1141 Y)
H = Y (0.135017 + 0.59482 Y)
For VI >100, VI is determined by the formula:
VI = {((antilog N) – 1)/0.00715} + 100, (8.7.8)
where N = (log H – log U)/ log Y. H is determined from the standard table.
If the kinematic viscosity of the oil product at 100°C above 70 m/s, H calculated by the formula: H = 0.1684 Y2 + 11.85 Y – 97
It is much easier to determine the viscosity index by nomograms (Appendix 7).
Viscosity index is a common value included in the standards for oils in all countries of the world. The disadvantage of the viscosity index is that it characterizes the behavior of the oil only in the temperature range from 37.8 to 98.8°C (Fig 7.8.6).
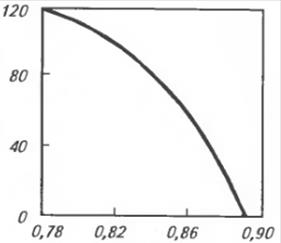
It was noticed that the density and viscosity of oils partially characterize their hydrocarbon composition. An appropriate indicator linking the density and viscosity of oils is proposed. This is called the viscosity-mass constant (VMC). Viscosity-mass constant can be calculated by the formula:
|

The depending on the chemical composition of the oil, its VMC can be from 0.75 to 0.90, and the higher the oil VMC, the lower its viscosity index (Fig. 7.8.7).

 |
In the area of low temperatures, petroleum products change their structure and are characterized by a yield point, plasticity, thixotropy, or viscosity anomaly. The results of measuring the viscosity of petroleum products depend on their mixing, consumption, or both factors at the same time. Oils do not obey the Newtonian fluid flow law, according to which the change in viscosity must depend only on temperature.
Oil with an undisturbed structure has a significantly higher viscosity than after its destruction. The ability of a system to spontaneously restore its structure is called thixotropy. When the flow rate of the oil product increases, that is the velocity gradient (section 1 of the curve), the oil structure is destroyed (Fig. 8.7.8), and the viscosity of the substance decreases and reaches a certain minimum (section 2). As the velocity gradient increases until a turbulent flow occurs, the viscosity increases again (section 3).


 |
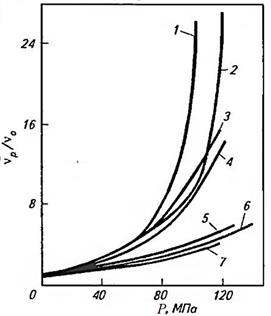
The viscosity of liquids, including petroleum products, depends on the external pressure.
Changing the viscosity with increasing pressure is of great practical importance, since high pressures can occur in some friction nodes.
The dependence of viscosity on pressure for some oils is illustrated by curves (Fig. 8.7.9).
 2020-05-25
2020-05-25 134
134