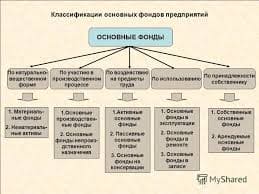
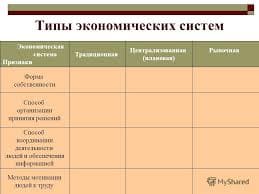
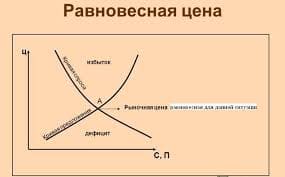
hydrogen bond - водородная связь
interaction - взаимодействие
negative - отрицательный
to attract - притягивать
to account for - объяснять
chain - цепь
nucleus (pl. nuclei) - ядро
compared with - по сравнению
enthalpy - энтальпия
1. What is hydrogen bond?
2. Where does oxygen atom attract the electrons in the O-H bonds?
3. What is the strength of hydrogen bond?
4. Why can’t hydrogen atom shield the nucleus?
5. How many lone electron pairs does each oxygen atom have?
Exercise 3. Put questions to the bold-typed words.
1. The hydrogen atom has no inner shells of electrons.
2. Hydrogen bonding has significant effects on physical properties.
3. There is an electrostatic interaction between the hydrogen proton and a lone pair of electrons on an oxygen atom in a neighboring molecule.
4. Hydrogen bond is caused by the electron-withdrawing properties.
5. Hydrogen bonds are not purely electrostatic.
6. Hydrogen bonding occurs between bases.
7. These bases are in the chains of DNA.
8. Hydrogen bond has a covalent character.
Exercise 4. Give the equivalents for the following words and make up sentences with them: atom, electron, bond, covalent, shell, oxygen, interaction, proton, boiling point, chain, nucleus, to attract, attraction, lone.
Exercise 5. Put the following words in the gaps: lone, electrons, shell, to shield, DNA, properties, chain.
1. … are particles that are contained in the atoms of any substances.
2. Boron atom in outer … has three electrons.
3. Aluminium has amphoteric ….
4. … is a carrier of genetic properties of living organisms.
5. Hydrogen atom has a … electron.
6. He … his child with his own body.
7. Yesterday my brother gave me a beautiful golden ….
Exercise 6. Give the explanation for the following words: shell, electron, chain, DNA, proton, hydrogen bond, covalent bond, oxygen.
Exercise 7. Put the prepositions into the gaps: between, in, to, on, of, by, during,
1. Hydrogen bondis a type … electrostatic interaction … molecules.
2. It can be regarded as a strong dipole-dipole attraction caused … the electron-withdrawing properties … the electronegative atom.
3. … the water molecule the oxygen atom attracts the electrons.
4. The substance was placed … the substrate.
5. Each oxygen atom has two lone pairs and can make hydrogen bonds... two different hydrogen atoms.
6.... the experiment there were no changes.
Exercise 8. Find and correct the mistakes.
 2015-05-13
2015-05-13 389
389