Lecture № 3.
1. Silicaoxygen motives in structures of silicates.
2. Job N.V.Belov.
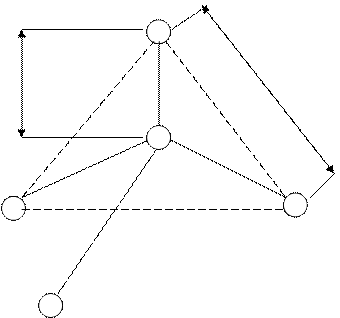
Xonotlite a tape is basis of structure many hydrosilicate of calcium.
Xonotlite a tape - doubling wollastonite of a chain.
Silicates represent the special class of inorganic connections, basic which structural unit are isolated or connected with each other silicaoxygen tetrahedral of group [SiO4] 4- (orthosilicates ions)
0,162нм 0,264нм
 | |||
 | |||
Figure 1. - Average parameters tetrahedral of group [SiO4] 4- in silica.
The sizes tetrahedral of group in silicates depending on ratio of connection and degree it electrovalence change in rather narrow limits, in particular, lengths of connection Si – O about makes on the average 0,162nm, and distance between atoms of oxygen 0,264 nm.
By one of characteristic properties tetrahedral of group [SiO4] 4- is their ability to be united with each other through common atoms of oxygen named bridge such association can occur to education of communication(connection) Si - About - Si. only by generalizations of tops tetrahedron (instead of edges or sides), and everyone tetrahedron group can have with next one, two, three or all four tops (that is general(common) atoms of oxygen).
Due to this are created all мА various on character of a combination mutually connected tetrahedral of groups [SiO4] 4-, forming various on structure and structure large complexes, which in structure silicate name silicaoxygen by motives or radicals. The modern classification is based on character these silicaoxygen of motives.
Except for silicaoxygen of groups structure silicate, which differ by rather complex(difficult) chemical structure, includes many other ions, in particular: Li, Ne, K, Be, Mg, Ca, Ti, Mn, Fe, Zn, B, Ba, O, H, F, and тд.
Some cation, first of all Al, B, Be have ability to simulate silicon in silicaoxygen a radical, that is partially isomorphic to replace an ion, entering in structure silicaoxygen of motive.
The special role in silicate is played by(with) aluminium, which in structure can replace a dual rule(situation).
The first period of study of structures silicate was connected to a name V.L. Bregga.
In the beginning 50 years N.V. Belov and his (its) school the new chains were open, the tapes and other motives in which basis lay not single tetrahedron [SiO4] 4-, and diorthogroup [Si 2O7] 6-.
В.Л. Брэггом were studied mainly silicate such cation as Mg2 +, Al2 3 + and as replacing them Fe2 +, Fe3 +. These cation in silicate structures have coordination number 6.
Н.В. Беловым were studied silicate with large cation: Na 2 +, Ca2 + etc. Basic silicaoxygen by unit is diorthogroup [Si 2O7] 6.
Example of chains with diorthogroup are wollastonite and rhodonite of a chain. In волластоните a basis of structure are the infinite columns from put on an edge octahedron. Everyone second octahedron is linked at once to both tetrahedron diorthogroup [Si2O7] 6-. However as height diorthogroup is a little bit more than an edge Са- octahedron, group [Si2O7] 6- is deformed, is delayed, coming nearer on the size to Са- octahedron.
The opposite edge of group is extended and the opportunity is created to connect among themselves two diorthogroup only by one octahedron
[SiO4] 4- fig. 1 in rhodonite, into which structure enter Ca, Mn - octahedron, chains an element of repeatability of this chain becomes complicated even more two diorto and one ortogroup also contain a fig. 1
The doubling wollastonite of a chain gives xonotlite a tape, which differs from amphibolic by that consists not from senary, and from octamerous. A radical xonotlite of a tape [Si 6O17] 10-. xonotlite the tape is a basis of structure many hydrosilicate calcium and enters into structure either as independent radicals or as the condensed layers. These hydrosilicate play the important role in processes hydration and hardening of cement. At high temperatures there is a break of tapes or layers, and the majority hydrosilicate turns in wollastonite.
In layered silicate except for the widely widespread grid from six-coal rings the turning out condensation amphibolic of tapes and characteristic for talc, micas, kaolinite, exists also grids formed(educated) by condensation xonotlite and more complex(difficult) tapes. In their structure as well as in a structure xonotlite of tapes, is precisely allocated diortogroup [Si 6O17] 10-.
diortogroup it is possible to allocate as and in frame silicate, at which are present only large катионы, and in some ring silicate. On the basis of research of the majority silicate with various cation Н.В. Белов has come to a conclusion, that the basic role in silicate structures belong cation to motives, by which only adapt кремнекислородные radicals. Between ions of oxygen environmental cation in structure silicate, the soft ions of silicon settle down. Each atom of silicon borrows(occupies) at the given moment only one of six next тетраэдрических of emptiness, as according to a rule Полинга, тетраэдры [SiO4] 4-, should not have general(common) edges. The atom of silicon can easily мигрировать from one тетраэдра in another, than and the opportunity of adaptation кремнекислородных of radicals to the basic motive of crystal structure is explained. If basic cation have the average sizes, the appropriate radical is under construction from classical тетраэдров [SiO4] 4-, if cation larger кремнекислородные radicals consist from диортогрупп [Si 2O7] 6-.
Questions:
1. What such кремнекислородные motives in structures силикатов?
2. What is basic кремнекислородной by unit?
3. Sizes тетраэдрической of group in силикатах, length of communication(connection) Si - About and distance between atoms of oxygen.
4. What such мостиковая communication(connection)?
5. What you know about jobs Н.В.Белова?
Кремнекислордный - Silicaoxygen
Ксонотлитовая лента - hydrosilicate of calcium
Гидросиликаты кальция - hydrosilicate of calcium
Удвоение волластонитовой цепочки - doubling wollastonite of a chain
Tетраэдрической – tetrahedral
Кратность - ratio
Ионность - electrovalence
Мостиковая связь – bridge bond
Такое объединение – such association
 2014-02-17
2014-02-17 959
959