Electronegativity is the relative ability of an atom to attract electrons in a covalent bond.
The electronegativity of an atom depends on its ability to attract electrons and its ability to hold onto electrons. Electronegativity increases across a period as the nuclear charge on the atoms increases but the shielding stays the same, so the electrons are more strongly attracted to the atom. Electronegativity decreases down a group as the number of shells increases, so shielding increases and the electrons are less strongly attracted to the atom.
An atom which has a high electronegativity is said to be electronegative; an atom which does not have a high electronegativity is said to be electropositive.
Electronegativities are relative; electronegativity has no units and is measured on a scale from 0.7 to 4.0. The electronegativities of some elements in the periodic table are shown below:
| H | He | ||||||||||||||||
| 2.1 | |||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | |||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 | |||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| 0.8 | 1.0 | 1.3 | 1.5 | 1.6 | 1.6 | 1.5 | 1.8 | 1.8 | 1.8 | 1.9 | 1.6 | 1.6 | 1.8 | 2.0 | 2.4 | 2.8 |
Note that the noble gases cannot be ascribed an electronegativity since they do not form bonds.
Electronegativity is a very useful concept for predicting whether the bonding between two atoms will be ionic, covalent or metallic.
Consider a covalent bond between two atoms A and B.

If both atoms have a similar electronegativity, both atoms attract the electrons with similar power and the electrons will remain midway between the two. The bond will thus be covalent - the electrons are shared between the two atoms.
Eg H (2.1) and H (2.1)
 a covalent bond
a covalent bond
If one atom is significantly more electronegative than the other, it attracts the electrons more strongly than the other and the electrons are on average closer to one atom than the other. The electrons are still shared, but one atom has a slight deficit of electrons and thus a slight positive charge and the other a slight surplus of electrons and thus a slight negative charge. Such a bond is said to be polar covalent.
Eg H (2.1) and O (3.0)
 a polar covalent bond
a polar covalent bond
A slight positive charge or negative charge on an atom is represented by a d+ or a d- symbol respectively.
If the difference between the two atoms is large, then the sharing of electrons is so uneven that the more electronegative atom has virtually sole possession of the electrons. The electrons are, in effect, not shared at all but an electron has essentially between transferred from one atom to the other. The more electropositive atom is positively charged and the more electronegative atom is negatively charged. The bonding is thus ionic.
Eg Na (0.9) and Cl (3.0)
 an ionic bond
an ionic bond
If both atoms are electropositive, neither has a great ability to attract electrons and the electrons do not remain localised in the bond at all. They are free to move, both atoms gain a positive charge and the bonding is metallic.
Eg Mg (1.2) and Mg (1.2)
 a metallic bond
a metallic bond
| Differences in electronegativity can be used to predict how much ionic or metallic character a covalent bond will have. |
Given suitable electronegativity data, it is thus possible to predict whether a bond between two atoms will be ionic, polar covalent, covalent or metallic.
If both atoms have electronegativities less than 1.6 - 1.9 then the bond is metallic.
If either atom has an electronegativity greater than 1.9 and the difference is less than 0.5 then the bond is covalent.
If either atom has an electronegativity greater than 1.9 and the difference is more than 0.5 but less than 2.1 then the bond is polar covalent.
If the difference is greater than 2.1 then the bond is ionic.
These rules are not perfect and there are notable exceptions; for example the bond between Si (1.8) and Si (1.8) is covalent but the bond between Cu (1.9) and Cu (1.9) is metallic. The bond between Na (0.9) and H (2.1) is ionic but the bond between Si (1.8) and F (4.0) is polar covalent. However as basic giudelines they are very useful provided that their limitations are appreciated.
| All bonds are assumed to be covalent in principle: differences in electronegativity can be used to predict how much ionic or metallic character a covalent bond will have. |
| Electronegativity differences show that bonds between non-identical atoms are all essentially intermediate in character between ionic and covalent. No bond is completely ionic, and only bonds between identical atoms are completely covalent. |
| Bonds between identical atoms cannot be ionic as there is no difference in electronegativity. They will therefore be either covalent or metallic. |
STATES OF MATTER
Matter can exist in one of three states; solid, liquid and gas. The state in which a certain substance is most stable at a given temperature depends on the balance between the kinetic energy of the particles, which depends on temperature, and the magnitude of the force of attraction between them.
i) Solids
In a solid, the particles are tightly packed together in a lattice. A lattice is an ordered and infinitely repeating arrangement of particles. The properties of solids are dominated by the forces in between these particles which cause them to attract each other and preserve this ordered arrangement.
A solid thus has a fixed volume and a fixed shape.
At all temperatures above absolute zero, the particles have kinetic energy. In a solid, however, this kinetic energy is not enough to cause the particles to fly apart, and nor is it enough to cause significant separation of the particles. The particles are thus restricted to rotational and vibrational motion; no translational motion of the particles with respect to each other is possible.
In a solid, the kinetic energy of the particles is not nearly enough to overcome the potential energy caused by their mutual attraction.
|
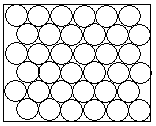
If a solid is heated, the kinetic energy of the particles increases, and they vibrate more. As they vibrate more, the bonds between the particles are weakened, some are broken and spaces appear between the particles. At this point the solid has melted.
ii) Liquids
In a liquid, the particles are by and large packed together in a lattice that extends across the range of 10 - 100 particles. However over a longer range the structure breaks down, and there is enough space between the particles for them to move from one cluster to another. The properties of liquids are still dominated by the forces between the particles, but these particles have enough kinetic energy to move between each other in the spaces that exist. There is thus short-range order but no long-range order.
A liquid has a fixed volume but no fixed shape.
The kinetic energy of the particles is now significant; it forces the particles apart to the extent that the spaces between them are often wider than the particles themselves. The particles are thus permitted some translational motion with respect to each other within these spaces. All solids will melt if they are heated strongly enough.
In a liquid, the kinetic energy of the particles is still not large enough to overcome their mutual attraction, but is nevertheless significant and must be taken into account.
|

iii) gases
In a gas, all the particles are in rapid and random motion, and thus behave independently of each other. There is no ordered arrangement of any kind, and the spaces between the particles are much larger than the size of the particles themselves. The properties of a gas are dominated by the kinetic energy of the particles; the attraction between them is not significant.
A gas has neither a fixed volume nor a fixed shape.
In a gas, the kinetic energy of the particles is much greater than the forces of attraction between them. Since the kinetic energy depends only on temperature, it follows that all gases at a similar temperature behave in a similar way. All liquids can be boiled if heated strongly enough.
|

IONIC STRUCTURES
 2017-11-30
2017-11-30 316
316