If there are three electron pairs on the central atom, the angle between the bonds is 120o.

| 
| 
|
Molecules which adopt this shape are said to be TRIGONAL PLANAR.
E.g. BF3, AlCl3, CO32-, NO3-
If one of these electron pairs is a lone pair, the bond angle is slightly less than 120o due to the stronger repulsion from lone pairs, forcing them closer together.
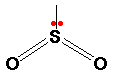 bond angle = 118o
bond angle = 118o
Molecules which adopt this shape are said to be BENT.
E.g. SO2, NO2-
C) Four electron pairs
If there are four bonded pairs on the central atom, the angle between the bonds is approx 109o.
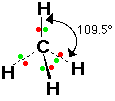
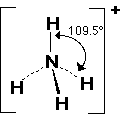

Molecules which adopt this shape are said to be TETRAHEDRAL.
E.g. CH4, SiCl4, NH4+, SO42-
If one of the electron pairs is a lone pair, the bond angle is slightly less than 109o, due to the extra lone pair repulsion which pushes the bonds closer together (approx 107o).

Molecules which adopt this shape are said to be TRIGONAL PYRAMIDAL.
E.g. NH3, PCl3
If two of the electron pairs are lone pairs, the bond angle is also slightly less than 109o, due to the extra lone pair repulsion (approx 104o).

Molecules which adopt this shape are said to be BENT.
E.g. H2O, OF2
D) Five electron pairs
If there are five bonded pairs on the central atom, the three bonds are in a plane at 120o to each other, the other 2 are at 90 o to the plane.

Molecules which adopt this shape are said to be TRIGONAL BYPRYMIDAL.
E.g. PF5, PCl5
 2017-11-30
2017-11-30 201
201