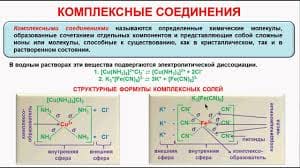
If there are six electron pairs on the central atom, the angle between the bonds is 90o.

Molecules which adopt this shape are said to be OCTAHEDRAL.
E.g. SF6
If there are 4 bonding pairs and 2 lone pairs, the bonded pairs are at 90o in the plane and the lone pairs at 180o. The angles are still exactly 90o because the lone pairs are opposite each other so their repulsion cancels out.

Molecules which adopt this shape are said to be SQUARE PLANAR.
E.g. XeF4, ClF4-
SUMMARY OF MOLECULAR SHAPES
| Valence shell electron pairs | Bonding pairs | Lone pairs | shape | Bond Angle (o) | ||
LINEAR

| ||||||
TRIGONAL PLANAR

| ||||||
BENT

| 115 - 118 |
TETRAHEDRAL

| 109.5 | |||||
TRIGONAL PYRAMIDAL

| ||||||
BENT

| 104.5 | |||||
TRIGONAL BIPYRAMIDAL

| 90 and 120 | |||||
OCTAHEDRAL

| ||||||
SQUARE PLANAR

|
 2017-11-30
2017-11-30 144
144