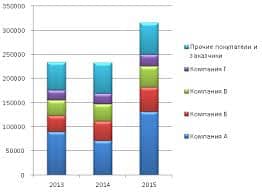
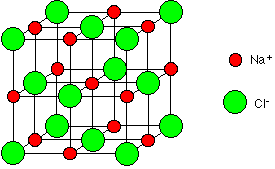
In sodium chloride, NaCl, each sodium ion is surrounded by six chloride ions and vice versa.
The diagram below shows the structure of sodium chloride. The pattern repeats in this way and the structure extends (repeats itself) in all directions over countless ions. You must remember that this diagram represents only a tiny part of the whole sodium chloride crystal.
|
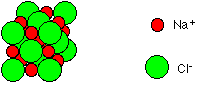 Each sodium ion attracts several chloride ions and vice versa so the ionic bonding is not just between one sodium and one chloride ion. There is a 3-D lattice.
Each sodium ion attracts several chloride ions and vice versa so the ionic bonding is not just between one sodium and one chloride ion. There is a 3-D lattice.
|
It could also be represented as follows:
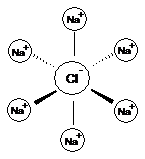

1. Melting and boiling point
The attraction between opposite ions is very strong. A lot of kinetic energy is thus required to overcome them and the melting point and boiling point of ionic compounds is very high.
In the liquid state, the ions still retain their charge and the attraction between the ions is still strong. Much more energy is required to separate the ions completely and the difference between the melting and boiling point is thus large.
| Compound | NaCl | MgO |
| Melting point/oC | ||
| Boiling point/oC |
The higher the charge on the ions, and the smaller they are, the stronger the attraction between them will be and the higher the melting and boiling points. In MgO, the ions have a 2+ and 2- charge and thus the attraction between them is stronger than in NaCl, so the melting and boiling points are higher.
2. Electrical Conductivity
Since ionic solids contain ions, they are attracted by electric fields and will, if possible, move towards the electrodes and thus conduct electricity. In the solid state, however, the ions are not free to move since they are tightly held in place by each other. Thus ionic compounds do not conduct electricity in the solid state. Ionic solids are thus good insulators.
In the liquid state, the ions are free to move and so can move towards their respective electrodes. Thus ionic compounds can conduct electricity in the liquid state.
3. Mechanical properties
Since ions are held strongly in place by the other ions, they cannot move or slip over each other easily and are hence hard and brittle.
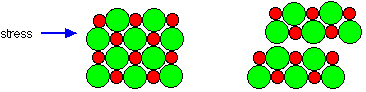
opposite ions attract like ions repel – structure breaks
METALLIC STRUCTURES
Bonding in metals
Metallic bonding is the attraction between cations and a sea of delocalised electrons. The cations are arranged to form a lattice, with the electrons free to move between them.
The structure of the lattice varies from metal to metal, and they do not need to be known in detail. It is possible to draw a simplified form of the lattice:
Example - magnesium
|
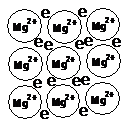
Properties of metals
a) Electrical conductivity: since the electrons in a metal are delocalised, they are free to move throughout the crystal in a certain direction when a potential difference is applied and metals can thus conduct electricity in the solid state. The delocalised electron system is still present in the liquid state, so metals can also conduct electricity well in the liquid state.
b) Melting and boiling point: although not generally as strong as in ionic compounds, the bonding in metals is relatively strong, and as a result the melting and boiling points of metals are relatively high.
| Metal | Na | K | Be | Mg |
| Melting point/ oC | ||||
| Boiling point/ oC |
Smaller ions, and those with a high charge, attract the electrons more strongly and so have higher melting points than larger ions with a low charge. Na has smaller cations than K so has a higher melting and boiling point. Mg cations have a higher charge than Na so has a higher melting and boiling point.
c) Other physical properties: Since the bonding in metals is non-directional, it does not really matter how the cations are oriented relative to each other. The metal cations can be moved around and there will still be delocalized electrons available to hold the cations together. The metal cations can thus slip over each other fairly easily. As a result, metals tend to be soft, malleable and ductile.
COVALENT STRUCTURES
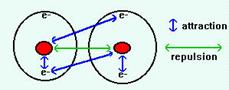
A covalent bond is a shared pair of electrons between two atoms. When a covalent bond is formed, two atomic orbitals overlap and a molecular orbital is formed. Like atomic orbitals, a molecular orbital can only contain two electrons. Overlap of atomic orbitals is thus only possible if both orbitals contain only one electron (normal covalent bond), or if one is full and the other empty (dative covalent bond).
Covalent bonding happens because the electrons are more stable when attracted to two nuclei than when attracted to only one:
1. Normal covalent bonds
An overlap between two orbitals, each containing one electron, is a normal covalent bond. The number of normal covalent bonds which an atom can form depends on its number of unpaired electrons. Some atoms, like carbon, promote electrons from s to p orbitals to create unpaired electrons.
1s 2s 2p
| F | ↑↓ | ↑↓ | ↑↓ | ↑↓ | ↑ |
F has 1 unpaired electron in a 2p orbital – forms one covalent bond
Eg hydrogen fluoride 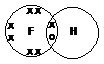
1s 2s 2p
| C | ↑↓ | ↑↓ | ↑ | ↑ |
Carbon rearranges slightly to make more unpaired electrons –
1s 2s 2p
| C | ↑↓ | ↑ | ↑ | ↑ | ↑ |
C has 4 unpaired electrons – forms four covalent bonds
Eg methane 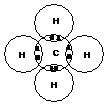
1. Dative covalent bonds
Any atom which has filled valence shell orbitals can provide both electrons for a dative covalent bond. This includes any element in groups V, VI, VII or 0 but is most common in N, O and Cl.
1s 2s 2p
| N | ↑↓ | ↑↓ | ↑ | ↑ | ↑ |
N has three unpaired electrons and one electron pair
Any atom which has empty valence shell orbitals can accept a pair of electrons for a covalent bond. This includes any element in groups I, II and III but is most common in Be, B and Al.
1s 2s 2p
| B | ↑↓ | ↑↓ | ↑ |
B promotes an electron from 2s to 2p to form 3 unpaired electrons:
1s 2s 2p
| B | ↑↓ | ↑ | ↑ | ↑ |
B has 3 unpaired electrons and an empty orbital
Eg BH3NH3 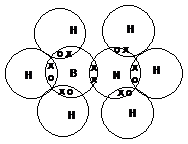
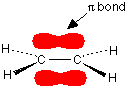
2. Sigma and pi bonds
Atomic orbitals can overlap in one of two ways:
If they overlap directly along the internuclear axis, as is most common, a s-bond is formed.
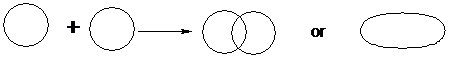
A s-bond is a bond resulting from direct overlap of two orbitals along the internuclear axis.
All single bonds between two atoms are s-bonds.
It is only possible to form one s-bond between two atoms, since another would force too many electrons into a small space and generate repulsion. If double bonds are formed, therefore, the orbitals must overlap in a different way.
If two orbitals overlap above and below (or behind and in front of) the internuclear axis, then a p-bond is formed.
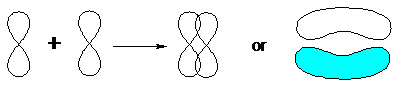
A p-bond is a bond resulting from overlap of atomic orbitals above and below the internuclear axis.
All double bonds consist of a s-bond and a p-bond.
All triple bonds consist of a s-bond and two p-bonds. If the first p-bond results from overlap above and below the internuclear axis, the second results from overlap behind and in front of the internuclear axis.
Note that p-bonds can only be formed by overlap of p-orbitals, since s-orbitals do not have the correct geometry.
p-bonds can also be represented by orbital diagrams.
Eg ethene:

| 
|
3. Strength of covalent bonds
Covalent bonds are in general strong. The smaller the atoms, the closer the electrons are to the two nuclei and the stronger the bond.
| Bond | Bond dissociation energy/ kJmol-1 |
| C-F | |
| C-Cl | |
| C-Br | |
| C-I |
4. Molecular, giant covalent and layered substances
Covalent bonding can result in three very different types of substance:
a) Molecular
In many cases, the bonding capacity is reached after only a few atoms have combined with each other to form a molecule. If no more covalent bonds can be formed after this, the substance will be made up of a larger number of discreet units (molecules) with no strong bonding between them.
Such substances are called molecular substances, and there are many examples of them: CH4, Cl2, He, S8, P4, O2, H2O, NH3 etc
The molecules are held together by intermolecular forces, which are much weaker than covalent bonds but are often strong enough to keep the substance in the solid or liquid state.
Example - Iodine

There are attractive forces between these molecules, known as intermolecular forces, but they are weak. In the gaseous state, the intermolecular forces are broken but the bonds within the molecule remain intact - they are not broken. The gas phase consists of molecules, not atoms.
Molecular substances have certain characteristic properties:
Melting and boiling point: these are generally low, since intermolecular forces are weak.
Intermolecular forces also decrease rapidly with increasing distance, so there is often little difference in the melting and boiling points.
| Substance | CH4 | H2O | H2 | He |
| Melting point /oC | -184 | -259 | -272 | |
| Boiling point /oC | -166 | -253 | -268 |
Electrical conductivity: There are no ions and no delocalised electrons, so there is little electrical conductivity in either solid or liquid state.
Other physical properties: The intermolecular forces are weak and generally non-directional, so most molecular covalent substances are soft, crumbly and not very strong.
b) Giant covalent
In some cases, it is not possible to satisfy the bonding capacity of a substance in the form of a molecule; the bonds between atoms continue indefinitely, and a large lattice is formed. There are no discrete molecules and covalent bonding exists between all adjacent atoms.
Such substances are called giant covalent substances, and the most important examples are C, B, Si and SiO2.
Example – diamond (diamond is an allotrope of carbon)
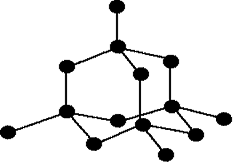
| Don't forget that this is just a tiny part of a giant structure extending on all 3 dimensions.

|
In giant covalent compounds, covalent bonds must be broken before a substance can melt or boil.
Giant covalent compounds have certain characteristic properties:
Melting and boiling point: these are generally very high, since strong covalent bonds must be broken before any atoms can be separated. The melting and boiling points depend on the number of bonds formed by each atom and the bond strength. The difference between melting and boiling points is not usually very large, since covalent bonds are very directional and once broken, are broken completely.
| Substance | C | Si | B | SiO2 |
| Melting point /oC | ||||
| Boiling point /oC |
Electrical conductivity: there are no ions or delocalised electrons, so there is little electrical conductivity in either solid or liquid state.
Other physical properties: since the covalent bonds are strong and directional, giant covalent substances are hard, strong and brittle.
Diamond is in fact the hardest substance known to man. For this reason it is used in drills, glass-cutting and styluses for turntables.
c) giant covalent layered
Some substances contain an infinite lattice of covalently bonded atoms in two dimensions only to form layers. The different layers are held together by intermolecular forces, and there are often delocalized electrons in between the layers. Examples of these structures are graphite and black phosphorus.
Example - graphite
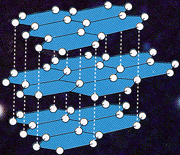 or
or 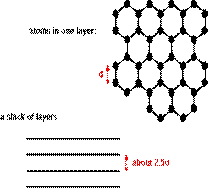
In graphite, each carbon atom is bonded to three others. The spare electron is delocalized and occupies the space in between the layers. All atoms in the same layer are held together by strong covalent bonds, and the different layers are held together by intermolecular forces.
A number of characteristic properties of graphite result from this structure:
Electrical conductivity: due to the delocalised electrons in each plane, graphite is a very good conductor of electricity in the x and y directions, even in the solid state (unusually for a non-metal). However, since the delocalisation is only in two dimensions, there is little electrical conductivity in the z direction (i.e. perpendicular to the planes).
Density: graphite has a much lower density than diamond (2.25 gcm-3) due to the relatively large distances in between the planes.
Hardness: graphite is much softer than diamond since the different planes can slip over each other fairly easily. This results in the widespread use of graphite in pencils and as an industrial lubricant.
 2017-11-30
2017-11-30 291
291