| SUBSTANCE | Nature of bonding | Physical properties |
| IONIC Eg NaCl | Attraction between oppositely charged ions. Infinite lattice of oppositely charged ions in three dimensions | High mpt, bpt Good conductors in liquid state Poor conductors in solid state Hard, strong, brittle |
| METALLIC Eg Mg | Attraction between cations and delocalised electrons. Infinite lattice of cations in three dimensions, with delocalized electrons in the spaces | High mpt, bpt Good conductors in solid state Good conductors in liquid state Strong, malleable |
| GIANT COVALENT Eg diamond | Infinite lattice of atoms linked by covalent bonds in three dimensions. Covalent bonds are pairs of electrons shared between two atoms | Very high mpt, bpt Poor conductors in solid state Poor conductors in liquid state Hard, strong, brittle |
| MOLECULAR Eg I2 | Discrete molecules. Atoms in molecule linked by covalent bonds. (s or p, normal or dative) Weak intermolecular forces between molecules. | Low mpt, bpt Poor conductors in solid state Poor conductors in liquid state Soft, weak, powdery |
| GIANT COVALENT LAYERED Eg graphite | Infinite lattice of atoms linked by covalent bonds in two dimensions to form planes. Planes held together by intermolecular forces. Delocalised electrons in between layers | High mpt, bpt Good conductors parallel to planes Poor conductors perpendicular to planes Soft |
Don’t forget to learn the structures of
- Sodium chloride
- Iodine
- Diamond
- Graphite
MOLECULAR SHAPES
When an atom forms a covalent bond with another atom, the electrons in the different bonds and the non-bonding electrons in the outer shell all behave as negatively charged clouds and repel each other. In order to minimise this repulsion, all the outer shell electrons spread out as far apart in space as possible.
Molecular shapes and the angles between bonds can be predicted by the VSEPR theory
VSEPR = valence shell electron pair repulsion
VSEPR theory consists of two basic rules:
i) All s-bonded electron pairs and all lone pairs arrange themselves as far apart in space as is possible. p-bonded electron pairs are excluded.
ii) Lone pairs repel more strongly than bonding pairs.
These two rules can be used to predict the shape of any covalent molecule or ion, and the angles between the bonds.
A) 2 electron pairs
If there are two electron pairs on the central atom, the angle between the bonds is 180o.
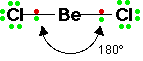
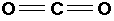
Molecules which adopt this shape are said to be LINEAR.
E.g. BeCl2, CO2
 2017-11-30
2017-11-30 163
163