There are no covalent bonds between molecules in molecular covalent compounds. There are, however, forces of attraction between these molecules, and it is these which must be overcome when the substance is melted and boiled. These forces are known as intermolecular forces. There are three main types of intermolecular force:
1. Van der Waal's forces
Consider a molecule of oxygen, O2.

The electrons in this molecule are not static; they are in a state of constant motion. It is therefore likely that at any given time the distribution of electrons will not be exactly symmetrical - there is likely to be a slight surplus of electrons on one of the atoms.

This is known as a temporary dipole. It lasts for a very short time as the electrons are constantly moving. Temporary dipoles are constantly appearing and disappearing.
Consider now an adjacent molecule. The electrons on this molecule are repelled by the negative part of the dipole and attracted to the positive part, and move accordingly.
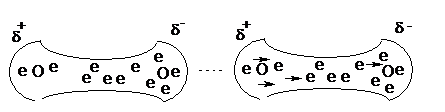
This is known as an induced dipole. There is a resulting attraction between the two molecules, and this known as a Van der Waal's force.
Van der Waal's forces are present between all molecules, although they can be very weak. They are the reason all compounds can be liquefied and solidified. Van der Waal's forces tend to have strengths between 1 kJmol-1 and 50 kJmol-1.
The strength of the Van der Waal's forces in between molecules depends on two factors:
a) the number of electrons in the molecule
The greater the number of electrons in a molecule, the greater the likelihood of a distortion and thus the greater the frequency and magnitude of the temporary dipoles. Thus the Van der Waal's forces between the molecules are stronger and the melting and boiling points are larger.
Eg noble gases:
| Substance | He | Ne | Ar | Kr |
| Number of electrons | ||||
| Melting point/oC | -272 | -252 | -189 | -157 |
| Boiling point/oC | -269 | -250 | -186 | -152 |
Eg alkanes:
| Substance | CH4 | C2H6 | C3H8 | C4H10 |
| Number of electrons | ||||
| Melting point/oC | -182 | -183 | -190 | -138 |
| Boiling point/oC | -164 | -88 | -42 |
a) Surface area of the molecules
The larger the surface area of a molecule, the more contact it will have with adjacent molecules. Thus the greater its ability to induce a dipole in an adjacent molecule and the greater the Van der Waal's forces and melting and boiling points.
This point can be illustrated by comparing different molecules containing a similar number of electrons:
| Substance | Kr | Cl2 | CH3CH(CH3)CH3 | CH3CH2CH2CH3 |
| Number of electrons | ||||
| Melting point/oC | -157 | -101 | -159 | -138 |
| Boiling point/oC | -152 | -35 | -12 |
CH3CH(CH3)CH3 CH3CH2CH2CH3
methylpropane butane

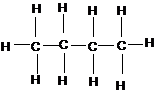
Note that butane has a larger surface area than 2-methylpropane, although they have the same molecular formula (C4H10). Straight-chain molecules always have higher boiling points than their isomers with branched chains.
2. Dipole-dipole bonding
Temporary dipoles exist in all molecules, but in some molecules there is also a permanent dipole.
Most covalent bonds have a degree of ionic character resulting from a difference in electronegativity between the atoms. This results in a polar bond and a dipole.


In many cases, however, the presence of polar bonds (dipoles) does not result in a permanent dipole on the molecule, as there are other polar bonds (dipoles) in the same molecule which have the effect of cancelling each other out. This effect can be seen in a number of linear, trigonal planar and tetrahedral substances:



CO2 BF3 CCl4
In all the above cases, there are dipoles resulting from polar bonds but the vector sum of these dipoles is zero; i.e. the dipoles cancel each other out. The molecule thus has no overall dipole and is said to be non-polar.
Non-polar molecules are those in which there are no polar bonds or in which the dipoles resulting from the polar bonds all cancel each other out. The only intermolecular forces that exist between non-polar molecules are temporary-induced dipole attractions, or Van der Waal’s forces.
In other molecules, however, there are dipoles on the molecule which do not cancel each other out:



CHCl3 SO2 NH3
In all the above cases, there are dipoles resulting from polar bonds whose vector sum is not zero; i.e. the dipoles do not cancel each other out. The molecule thus has a permanent dipole and is said to be polar.
Polar molecules are those in which there are polar bonds and in which the dipoles resulting from the polar bonds do not cancel out.
In addition to the Van der Waal's forces caused by temporary dipoles, molecules with permanent dipoles are also attracted to each other by dipole-dipole bonding. This is an attraction between a permanent dipole on one molecule and a permanent dipole on another.



Dipole-dipole bonding usually results in the boiling points of the compounds being slightly higher than expected from temporary dipoles alone; it slightly increases the strength of the intermolecular bonding.
The effect of dipole-dipole bonding can be seen by comparing the melting and boiling points of different substances which should have Van der Waal's forces of similar strength:
| Substance | Cl2 | HBr | CH3CH(CH3)CH3 | CH3COCH3 |
| Number of electrons | ||||
| Permanent dipole | NO | YES | NO | YES |
| Melting point/oC | -101 | -88 | -159 | -95 |
| Boiling point/oC | -45 | -67 | -73 | -44 |
3. Hydrogen bonding
In most cases as seen above, the presence of permanent dipoles only makes a slight difference to the magnitude of the intermolecular forces. There is one exceptional case, however, where the permanent dipole makes a huge difference to the strength of the bonding between the molecules.
Consider a molecule of hydrogen fluoride, HF. This clearly has a permanent dipole as there is a large difference in electronegativity between H (2.1) and F (4.0). The electrons in this bond are on average much closer to the F than the H:

The result of this is that the H atom has on almost no electron density around its nucleus at all and is therefore very small. The H atom is therefore able to approach electronegative atoms on adjacent molecules very closely and form a very strong intermolecular dipole-dipole bond.

This is known as hydrogen bonding. It is only possible if the hydrogen atom is bonded to a very electronegative element; i.e. N, O or F. It is not fundamentally different from dipole-dipole bonding; it is just a stronger form of it.
A hydrogen bond can be defined as an attraction between an electropositive hydrogen atom (ie covalently bonded to N, O or F) and an electronegative atom on an adjacent molecule.
Examples of substances containing hydrogen bonds are HF, H2O, NH3, alcohols, carboxylic acids, amines, acid amides and urea.

a) Effect on boiling point
The effect of hydrogen bonding on melting and boiling points of substances is huge, unlike other dipole-dipole bonds. Many substances containing hydrogen bonds have much higher boiling points than would be predicted from Van der Waal's forces alone.
| Substance | CH3OCH3 | CH3CH2OH | CH3CH2CH2CHO | CH3CH2COOH |
| Structure | 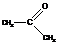
|

| 
| 
|
| Number of electrons | ||||
| Hydrogen bonding | NO | YES | NO | YES |
| Melting point/oC | -95 | -117 | -81 | -21 |
| Boiling point/oC | -44 |
Another important series of trends are the boiling points of the hydrides of elements in groups V, VI and VII of the periodic table:
Group V: NH3, PH3, AsH3, SbH3
Group VI: H2O, H2S, H2Se, H2Te
Group VII: HF, HCl, HBr, HI
The boiling points of these graphs are shown graphically below:

In each case the hydride of period 2 shows a boiling point which is abnormally high
(H2O, NH3 and HF).
The general increase in boiling point down the groups result from the increase in Van der Waal's forces which results from an increasing number of electrons in the molecules. There are permanent dipoles but they are not very strong.
The abnormally high boiling points of H2O, NH3 and HF are a result of hydrogen bonding between the molecules. Thus results in very strong intermolecular forces between the molecules despite the fact that the Van der Waal's forces are weaker than in the other hydrides.
b) other effects of hydrogen bonding
The effects of hydrogen bonding on the physical properties of a substance are not restricted to elevated melting and boiling points; it can influence the properties of substances in other ways:
The low density of ice. This is due to hydrogen bonding. In ice, the water molecules arrange themselves in such a way as to maximise the amount of hydrogen bonding between the molecules. This results in a very open hexagonal structure with large spaces within the crystal. This accounts for its low density.
When the ice melts, the structure collapses into the open spaces and the resulting liquid, despite being less ordered, occupies less space and is thus more dense.
Thus ice floats on water.
The helical nature of DNA. This is also due to hydrogen bonding. Molecules of DNA contain N-H bonds and so hydrogen bonding is possible. The long chains also contain C=O bonds and the H atoms can form a hydrogen bond with this electronegative O atom. This results in the molecule spiralling, as the C=O bonds and the N-H bonds approach each other.
This is an example of an intramolecular hydrogen bond, where the attraction is between a hydrogen atom and an electronegative atom on the same molecule. This must be distinguished from intermolecular hydrogen bonding, in which the attraction is between a hydrogen atom and an electronegative atom on an adjacent molecule.
 2017-11-30
2017-11-30 222
222